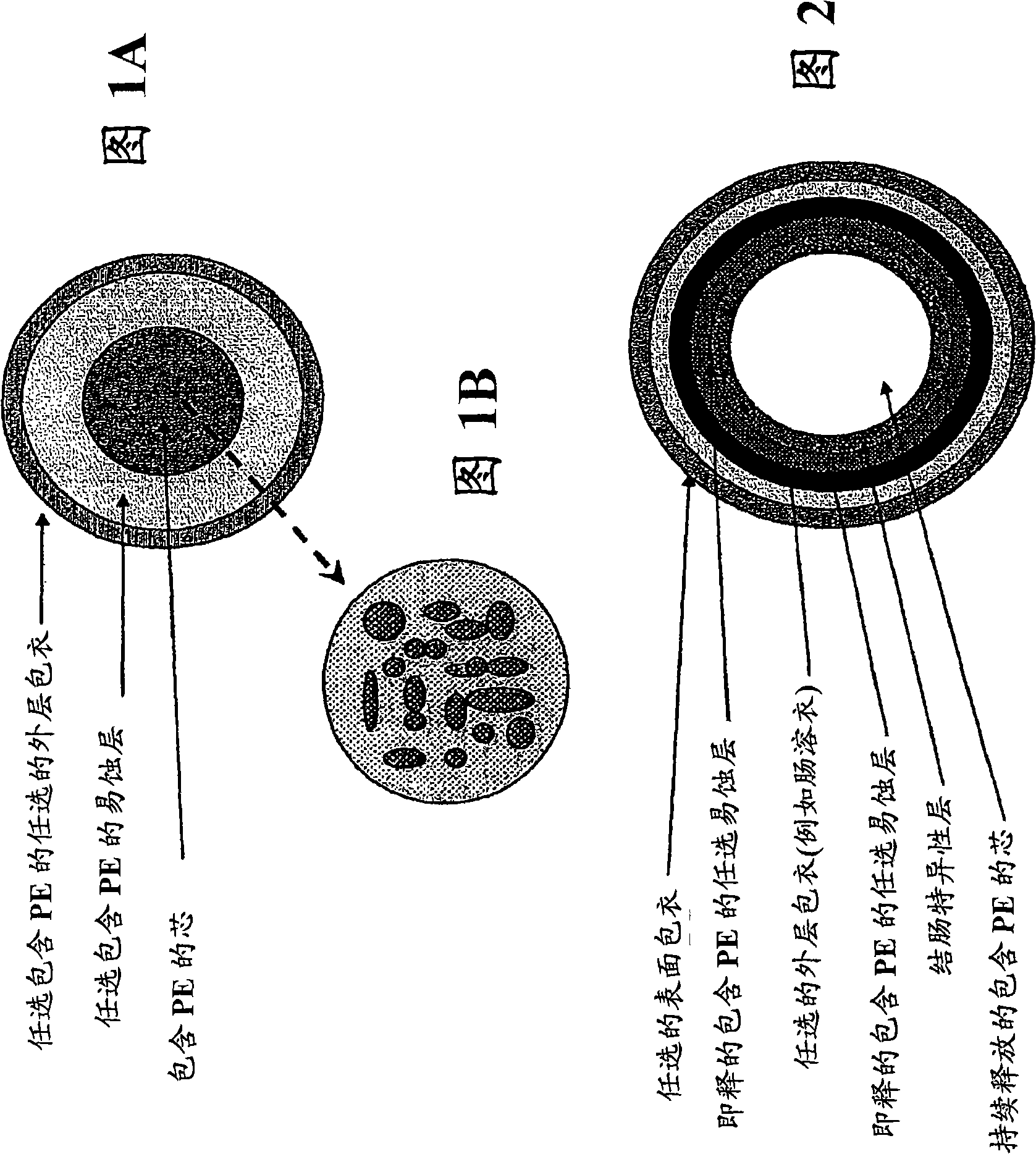
Sustained release pharmaceutical formulation comprising phenylephrine
A phenylephrine, pharmaceutical technology, applied in the field of salt pharmaceutical composition, can solve the problem of unsatisfied continuous availability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0140] The preferred method of manufacture is direct compression using a two-layer rotary tablet press. A preferred composition is a bilayer tablet having two distinct layers: a sustained release layer and an immediate release layer. For extended-release tablets, first mill phenylephrine HCl through a laboratory-size conical mill using a 20-mesh screen. Hydroxypropylcellulose Klucel EXF, Hydroxypropylcellulose Klucel GXF, sodium carboxymethylcellulose and one-third of microcrystalline cellulose were passed through the same conical mill. The ground material was then mixed in an appropriately sized PK blender for 5 minutes. The remaining microcrystalline cellulose was added to the blender and blended for an additional 5 minutes. Magnesium stearate was manually screened through a 30 mesh screen, added to the blend and blended for an additional 3 minutes and discharged into a suitably labeled container.
[0141] For immediate-release tablets, phenylephrine hydrochloride and lor...
Embodiment 1
[0153] Example 1. Bioavailability of Phenylephrine
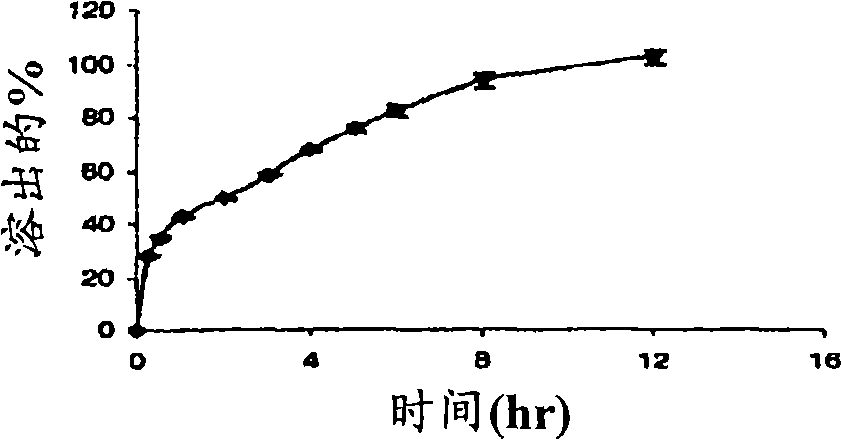
[0154] The following study was performed to examine the absorption behavior of phenylephrine in the GI tract. Administration to healthy male and nonpregnant, nonlactating healthy female subjects: via Enterion TM Capsules (Protocol A - 9 subjects) 10 mg phenylephrine hydrochloride, 10 mg Sudafed PE delivered to the colon TM (Protocol B - 8 subjects) or via Enterion TM Capsules (Schedule C - 8 subjects) delivered 30 mg of phenylephrine hydrochloride to the colon, all regimens administered orally after an overnight fast. enter TM Capsules (Pharmaceutical Profiles Ltd, UK) allow for the orchestration of drug release at desired locations within the GI tract. To determine that the capsules have reached the desired location, the formulations of regimens A and C are administered orally with 210 mL of water followed by a radiolabeled drink containing 4 MBq 99m-Tc-diethylenetriaminepentaacetic acid (DTPA) in 30 mL of water so that...
Embodiment 2
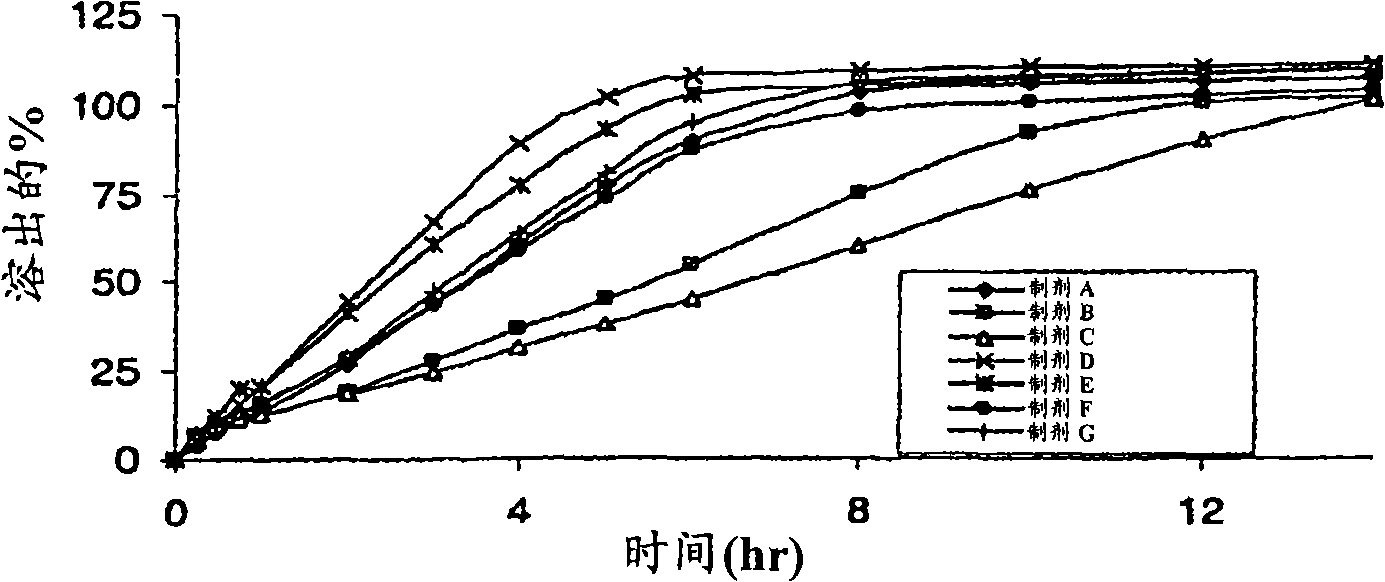
[0194] Example 2. Preparation of Exemplary Formulations A-G and Determination of Dissolution Profiles
[0195] The dissolution profiles of exemplary formulations A-G (detailed in Table 2) were tested using a USP I dissolution apparatus set at 75 rpm over a period of 14 hours. Dissolution studies were performed at 37°C ± 0.5°C with 900ml of water buffered with 0.5mM phosphate buffer, pH 7.4. At each time interval, samples of the solution were analyzed by HPLC at 215 nm to determine the percent phenylephrine dissolved. Table 15 shows the three average dissolution results. and, image 3 Dissolution profiles are shown.
[0196] Table 15.
[0197]
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com