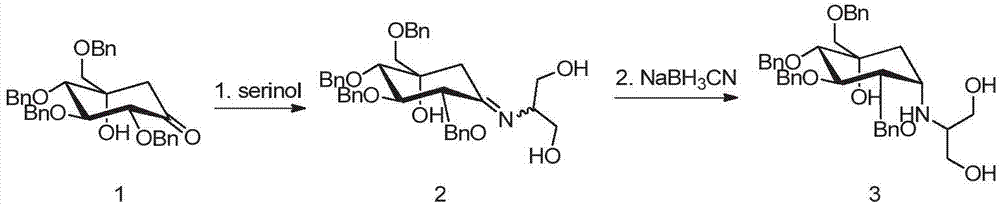
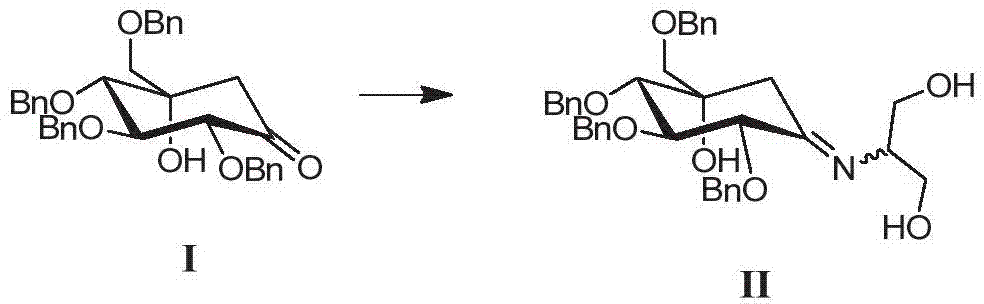
A kind of preparation method of tetrabenzyl voglibose
A compound and reducing agent technology, applied in the preparation of organic compounds, amino hydroxyl compounds, sulfonates, etc., can solve the problems of potential safety hazards, the impact of voglibose purity, and the expensive sodium cyanoborohydride , to achieve the effect of improving production safety, reducing raw material costs, and being suitable for industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1 (1S)-(1(hydroxyl), 2,4,5 / 1,3)-2,3,4-tri-oxo-benzyl-5-[(2-hydroxyl-1-(hydroxymethyl Base) ethyl) amino] -1-carbon-benzyloxymethyl-1,2,3,4-cyclohexanetetraol benzenesulfonate (compound IV)
[0034]Add 141.0g of ketone compound I, 46.5g of serinol, and 1020mL of absolute ethanol in sequence into a 3L three-necked flask, stir, add 0.73mL of glacial acetic acid, heat in a water bath, and raise the temperature to 33-37°C. When the solid is basically dissolved, start Timing; keep warm at 33-37°C, react for about 3.0h-3.5h to obtain compound II; add 55.0g potassium borohydride several times in a small amount, after the addition is complete, control the temperature at 33-37°C, and react for 5-8h; Add 710mL of water, stir for 20-25 minutes, concentrate under reduced pressure at 40°C to remove most of the solvent, stop distillation, add 1410mL of ethyl acetate to the residue, then add 710mL of water, stir for 15-20min, separate the liquids, and keep the organic layer. E...
Embodiment 2
[0036] The preparation of embodiment 2 compound IV
[0037] Add 141.0g of Compound I, 58.1g of serinol, and 1020mL of absolute ethanol to a 3L three-neck flask in sequence, stir, add 0.73mL of glacial acetic acid, heat in a water bath, and raise the temperature to 33-37°C. When the solid is basically dissolved, start timing ; keep warm at 33-37°C, react for about 3.0h-3.5h; add 55.0g of potassium borohydride in small amounts for several times, after the addition is complete, control the temperature at 33-37°C, and react for 5-8h; slowly add 710mL of water into the reaction bottle, stir After 20-25 minutes, concentrate under reduced pressure at 40°C to remove most of the solvent, stop the distillation, add 1410mL ethyl acetate to the residue, then add 710mL water, stir for 15-20min, separate the layers, keep the organic layer, and wash the water layer with 390mL× 2 Extract with ethyl acetate, combine the organic layers, wash the organic layer with 710mL×2 purified water in turn...
Embodiment 3
[0039] The preparation of embodiment 3 compound IV
[0040] Add 141.0g of compound I, 58.1g of serinol, and 1020mL of absolute ethanol into a 3L three-necked flask in turn, stir, add 0.8mL of propionic acid, heat in a water bath, and raise the temperature to 33-37°C. When the solid is basically dissolved, start timing ; keep warm at 33-37°C, react for about 3.0h-3.5h; add 55.0g of potassium borohydride in small amounts for several times, after the addition is complete, control the temperature at 33-37°C, and react for 5-8h; slowly add 710mL of water into the reaction bottle, stir After 20-25 minutes, concentrate under reduced pressure at 40°C to remove most of the solvent, stop the distillation, add 1410mL ethyl acetate to the residue, then add 710mL water, stir for 15-20min, separate the layers, keep the organic layer, and wash the water layer with 390mL× 2 Extract with ethyl acetate, combine the organic layers, wash the organic layer with 710mL×2 purified water in turn, sepa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com