Synthesis method of voglibose
A technique for voglibose and its synthesis method, which is applied in the direction of organic chemistry methods, chemical instruments and methods, sugar derivatives, etc., can solve the problems of complicated voglibose preparation process and high market price, and achieve low cost , simple operation, and the effect of saving production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
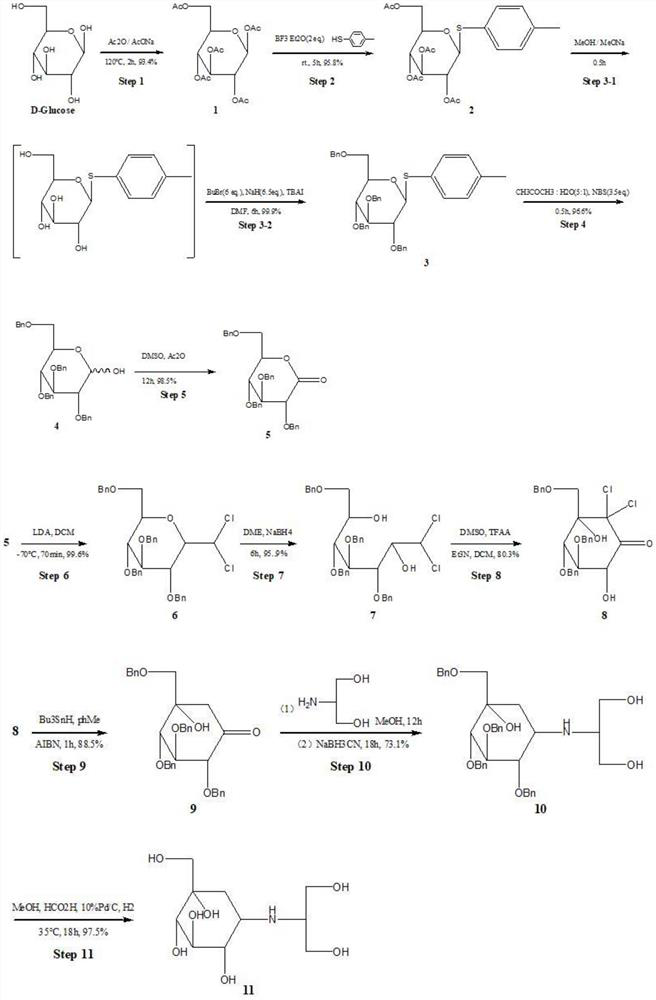
[0062] Step 1: Synthesis of Peracetylated Glucose
[0063] After mixing and grinding 19.8g of glucose monohydrate and 15g of sodium acetate, they were transferred to a three-neck flask, and 120mL of acetic anhydride (Ac 2 O), stirred at 120 ° C for 2h. Pour into 300g of ice water, stir vigorously, solids precipitate out, put it in an ice bath and let it stand for 2h. After suction filtration, the filter cake was washed with distilled water and recrystallized with absolute ethanol-cyclohexane to obtain 35.5 g of fully acetylated glucose with a yield of 71.3%.
[0064] In this step, different reaction conditions were tried. Such as: raw material ratio (glucose monohydrate: acetic anhydride = 1:5 or 1:6), reaction temperature (100°C, 140°C), reaction time (1h, 3h, 5h), the product yield obtained is 68 %-73%.
[0065] Step 2: Synthesis of 2,3,4,6-tetra-O-acetyl-β-D-glucophenanthin
[0066] Add 150mL of dichloromethane into the eggplant-shaped bottle, add 20g of peracetylated ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com