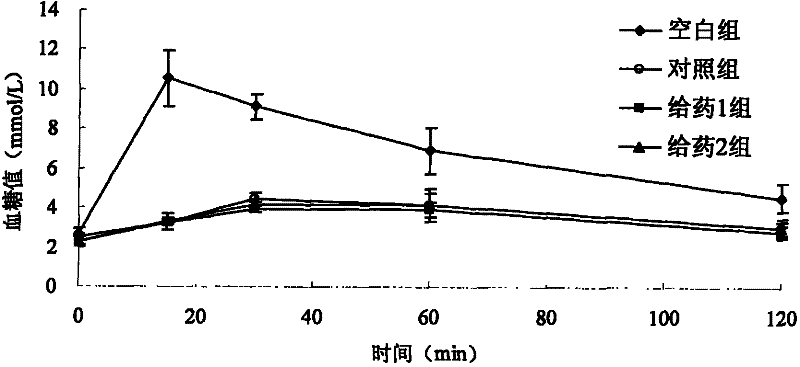
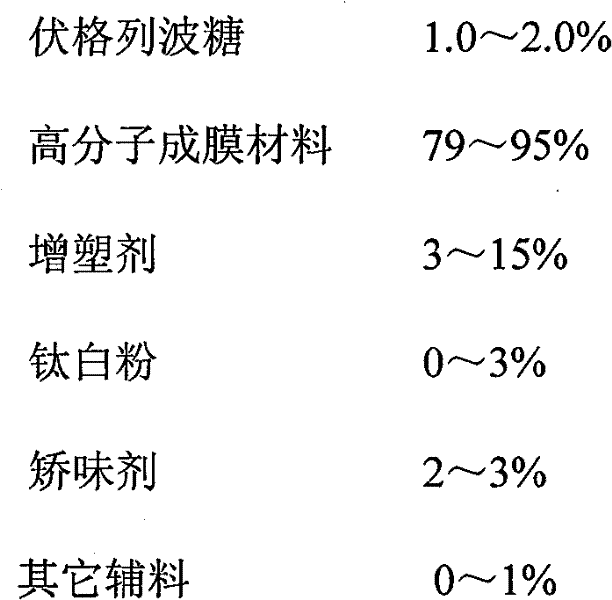
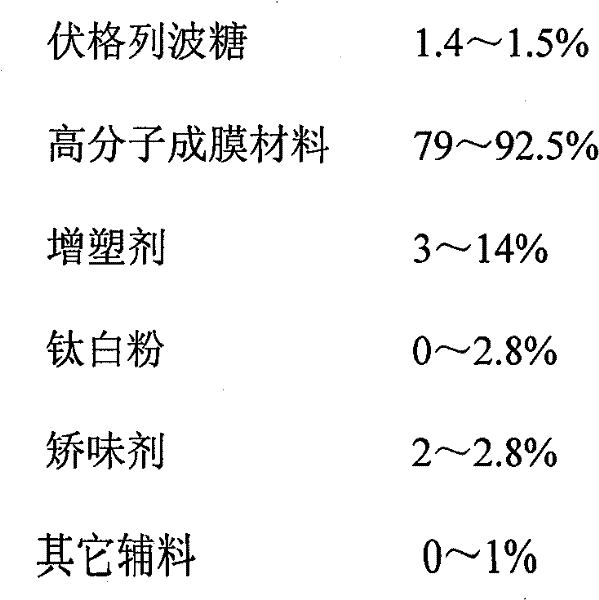
Voglibose film and preparation method thereof
A technology of voglibose and film preparations is applied in the directions of pharmaceutical formulations, medical preparations with non-active ingredients, and medical preparations containing active ingredients, etc. Achieve the effects of high added value, accelerated dissolution, and fast onset of action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] The prescription quantity is 5000 tablets (0.2mg specification) or 3333 tablets (0.3mg specification);
[0071] Voglibose 1g
[0072] Polyvinyl alcohol (PVA) 56g
[0073] Plasticizer Glycerin 10g
[0074] Titanium dioxide 2g
[0075] Aspartan 2g
[0076] Preparation:
[0077] Take voglibose and add 200ml of distilled water to dissolve, add glycerin and aspartame, stir to dissolve completely, then add titanium dioxide to make the dispersion uniform, then add PVA (molecular weight 130,000 Daltons), stir to form a solution, pass 80-mesh sieve to remove insoluble matter and vacuum defoaming. Then stretch the membrane solution on a stainless steel belt, dry at 80°C, emboss with an embossing roller, cut it into a certain size, peel it off from the stainless steel belt, and seal the package.
Embodiment 2
[0079] The prescription quantity is 7500 tablets (specification 0.2mg) or 5000 tablets (specification 0.3mg)
[0080] Voglibose 1.5g
[0081] Polymer film-forming material HPC 62.5g
[0082] Polymer film-forming material PEO 30g
[0083] Plasticizer PEG 400 3g
[0084] Acesulfame K 2g
[0085] Chlorophyll 1g
[0086] Dissolve the pigment chlorophyll in 10ml of ethanol, stir and mix with voglibose, HPC (molecular weight 80,000 Daltons), PEO (molecular weight 200,000 Daltons), PEG 400 and acesulfame potassium, heat to 100°C, melt , the embossing roller is extruded into a film and embossed, and cooled. After cutting into a certain size, it is sealed and packaged.
Embodiment 3
[0088] The prescription quantity is 50,000 tablets (0.3mg specification)
[0089] Voglibose 15g
[0090] Polymer film-forming material HPMC 50g
[0091] Plasticizer triacetin 18g
[0092] Plasticizer Tween-80 2g
[0093] Titanium dioxide 5g
[0094] Flavor xylitol 10g
[0095] Preparation:
[0096] Take voglibose and add 300ml of distilled water to dissolve, add glycerin, Tween-80 and xylitol, stir to dissolve, then add titanium dioxide to make the dispersion even, then add HPMC (molecular weight 20,000 Daltons), stir to form a solution, Pass through an 80-mesh sieve to remove insoluble matter, and place it overnight for vacuum degassing. Then spread the film solution on the plastic film, dry at 60°C, emboss with an embossing roller, cut into a certain size, peel off the plastic film, and seal the package.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com