A kind of voglibose tablet and preparation method thereof
A technology for voglibose and sugar tablets, which can be applied to pharmaceutical formulations, medical preparations without active ingredients, and medical preparations containing active ingredients, etc., can solve the problem of poor dissolution and affect the curative effect of voglibose and other problems, to achieve the effects of excellent dissolution, good disintegration, and improved efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
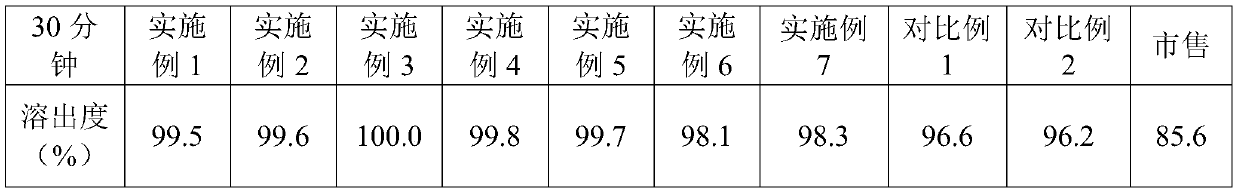
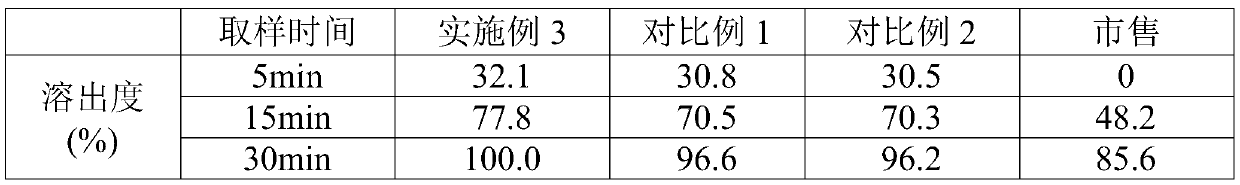
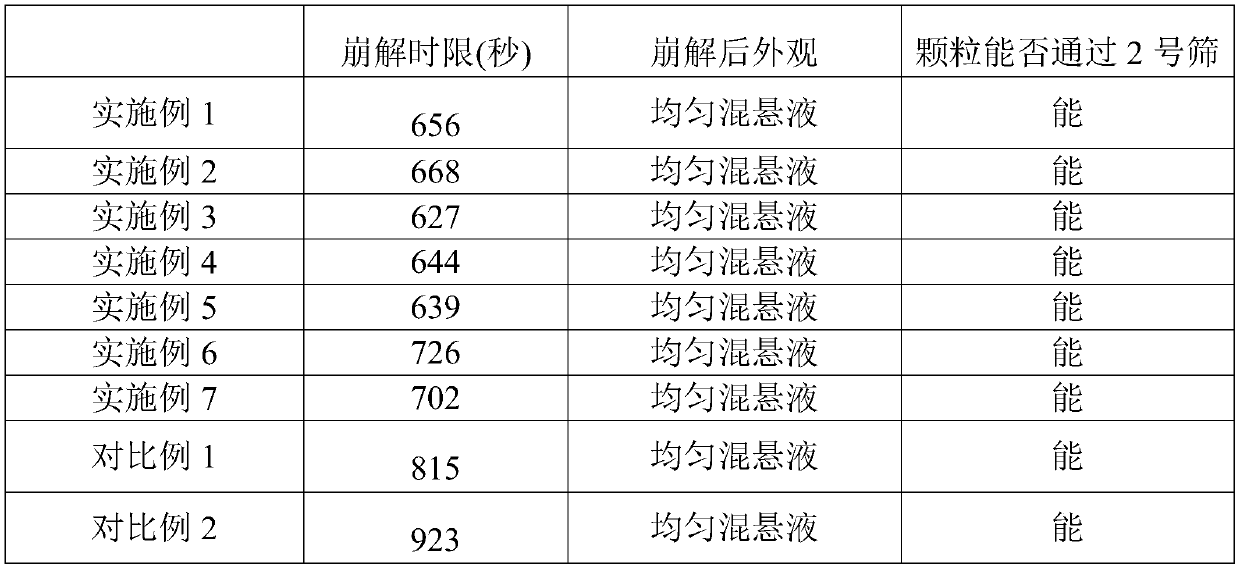
Examples
Embodiment 1
[0025] A voglibose tablet, comprising the following raw materials in parts by weight: 1 part of voglibose, 450 parts of lactose, 130 parts of starch, 20 parts of high-substituted hydroxypropyl fiber, 3 parts of magnesium stearate, and 0.1 part of propolis , 5 parts of sodium lauryl sulfate, 4 parts of polysorbate 80, 30 parts of co-solvents and 40 parts of disintegrants; said co-solvents are hydroxypropyl methylcellulose; Sodium Methylcellulose.
Embodiment 2
[0027] A voglibose tablet, comprising the following raw materials in parts by weight: 1 part of voglibose, 550 parts of lactose, 170 parts of starch, 30 parts of high-substituted hydroxypropyl fiber, 4 parts of magnesium stearate, and 0.5 part of propolis , 10 parts of sodium lauryl sulfate, 6 parts of polysorbate 80, 40 parts of cosolvents and 50 parts of disintegrants; said cosolvents are cyclodextrin and polyethylene glycol 6000, cyclodextrin and polyethylene glycol The weight ratio of alcohol 6000 is 1:1; the disintegrant is sodium carboxymethyl starch and microcrystalline cellulose, and the weight ratio of sodium carboxymethyl starch and microcrystalline cellulose is 1:1.
Embodiment 3
[0029] A voglibose tablet, comprising the following raw materials in parts by weight: 1 part of voglibose, 487.5 parts of lactose, 150 parts of starch, 25 parts of high-substituted hydroxypropyl fiber, 3.25 parts of magnesium stearate, and 0.3 part of propolis , 8 parts of sodium lauryl sulfate, 805 parts of polysorbate, 35 parts of co-solvent and 45 parts of disintegrating agent; The co-solvent is hydroxypropyl methylcellulose, cyclodextrin and polyethylene glycol 6000, hydroxyl The weight ratio of propyl methylcellulose, cyclodextrin and polyethylene glycol 6000 is 2:2:1; the disintegrating agent is croscarmellose sodium, sodium carboxymethyl starch and microcrystalline cellulose , The weight ratio of croscarmellose sodium, sodium carboxymethyl starch and microcrystalline cellulose is 1:2:2.
[0030] The preparation method of the voglibose tablet of above-mentioned embodiment 1~3, comprises the following steps:
[0031] S1, pretreatment: take lactose, starch, magnesium stea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com