A kind of preparation method of voglibose impurity I hydrochloride
A technology of voglibose and hydrochloride, which is applied in the field of medicine, can solve the problems of impurity I not yet prepared, and achieve the effects of improving drug safety, simple process flow, and improving quality standards
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
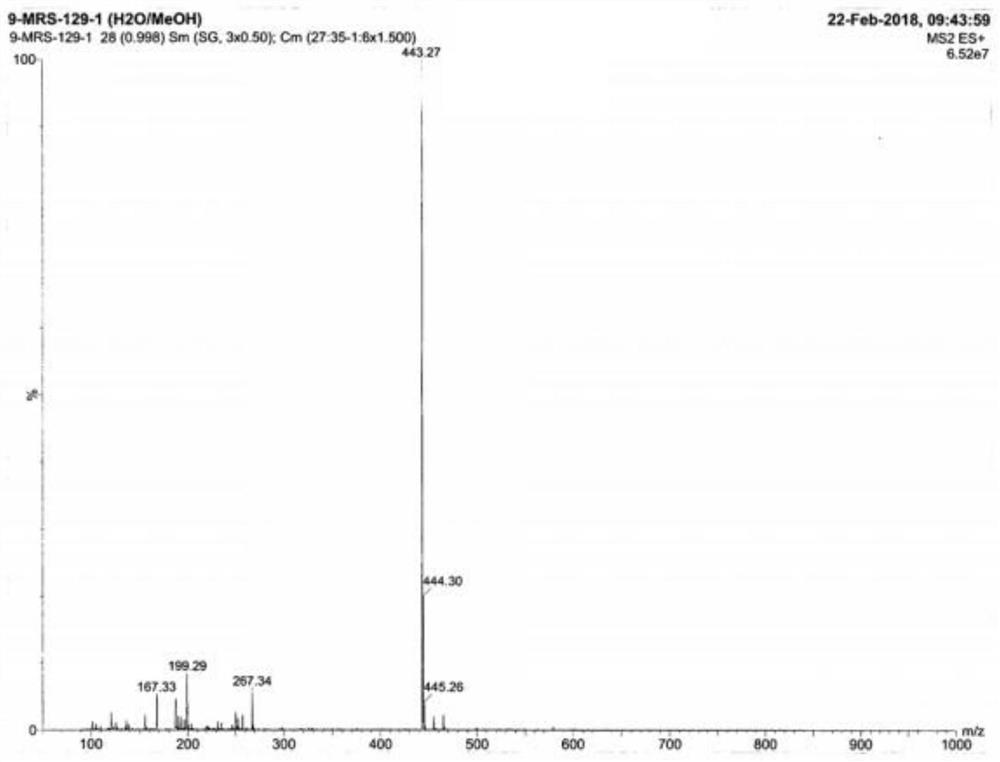
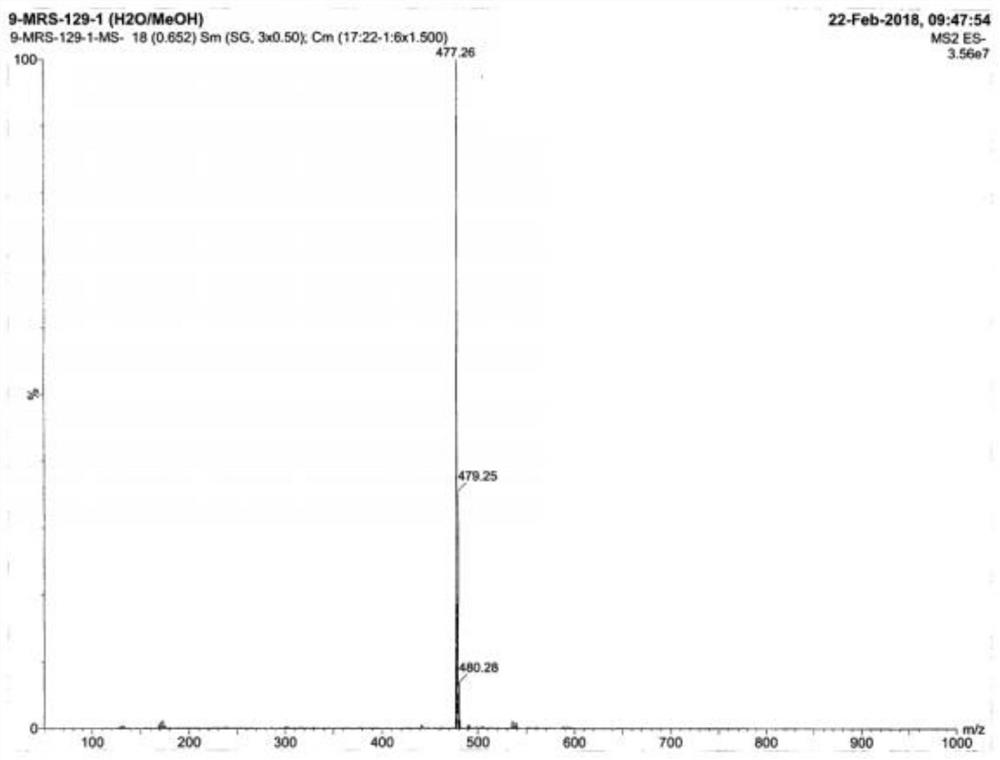
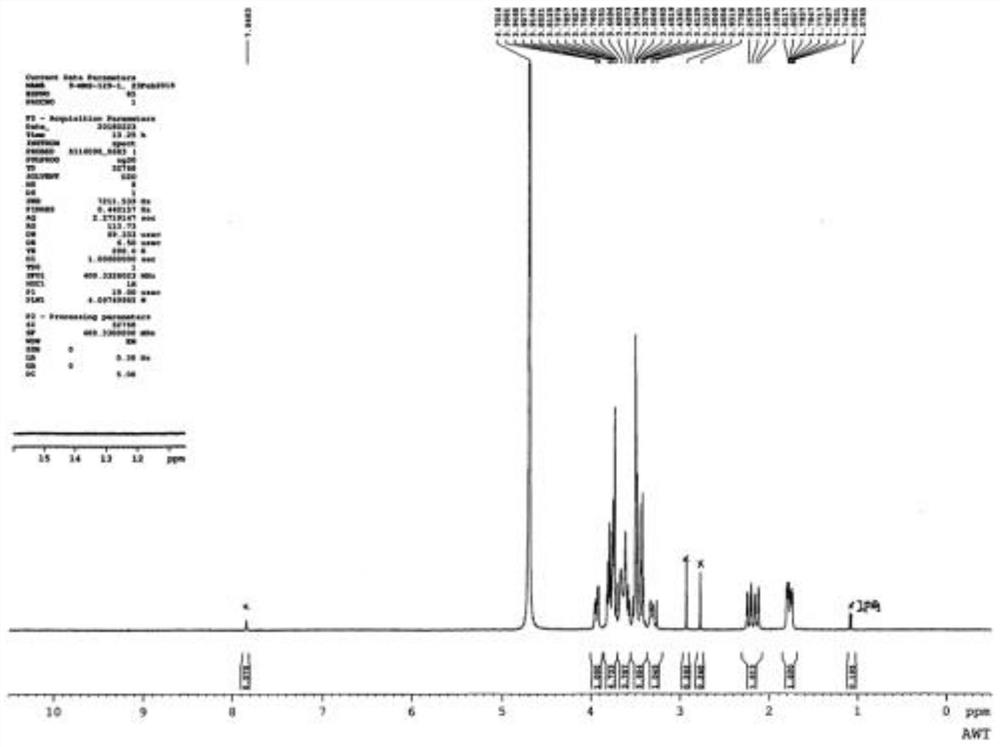
Image
Examples
Embodiment Construction
[0029] The present invention will be described in detail below with reference to the embodiments shown in the drawings. However, it should be noted that these embodiments do not limit the present invention, and those of ordinary skill in the art make functional, method, or structural improvements based on these embodiments. Equivalent changes or substitutions fall within the protection scope of the present invention.
[0030] The invention discloses a preparation method of voglibose impurity I hydrochloride, and the overall reaction process formula is:
[0031]
[0032] Specifically include the following steps:
[0033] Step 1: (S)-(Ethylene oxide methyl) carbamic acid tert-butyl ester and tetrabenzyl tetrabenzyl mycosylamine undergo epoxide amination to obtain the compound of formula III, namely tert-butyl ((2S)-3- Hydroxy-2-(((2S,3R,4S,5S)-2,3,4-tris(benzyloxy)-5-((benzyloxy)methyl)-5-hydroxycyclohexyl)amino)propane 基)tert-butyl carbamate;
[0034] The specific method is as follows...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com