A method for determining related substances in voglibose raw materials and preparations
A technology for voglibose and related substances, which is applied in the field of determination of related substances in voglibose raw materials and preparations, and can solve problems such as complex operations, affecting peak conditions, and affecting substance detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Method for determining impurities of related substances in voglipose capsules in high performance liquid phase: the APIs or preparations containing voglipose and related substances are dissolved in diluents and diluted to a concentration of 10mg / mL; Separation of voglipose and related substances with 1-phenyl-3-methyl-5-pyrazolinone and sodium hydroxide as derivatization reagents, octadecyl bonded porous silica gel as filler, and potassium dihydrogen phosphate buffer-acetonitrile mixed solution as the mobile phase; The dilution is water. The column is A C18 column, the mobile phase is pH 7.2 potassium dihydrogen phosphate buffer - acetonitrile (80:20), the flow rate is 0.7ml / min, the column temperature is 35 °C, the detection wavelength is 248 nm.
Embodiment 2
[0017] Method for determining impurities of related substances in voglipose capsules in high performance liquid phase: the APIs or preparations containing voglipose and related substances are dissolved in diluents and diluted to a concentration of 10mg / mL; Separation of voglipose and related substances with 1-phenyl-3-methyl-5-pyrazolinone and sodium hydroxide as derivatization reagents, octadecyl bonded porous silica gel as filler, and potassium dihydrogen phosphate buffer-acetonitrile mixed solution as the mobile phase; The dilution is water. The column is A C18 column, the mobile phase is pH 7.3 potassium dihydrogen phosphate buffer - acetonitrile (80:20), the flow rate is 0.8 ml / min, the column temperature is 30 °C, the detection wavelength is 250 nm.
Embodiment 3
[0019] Method for determining impurities of related substances in voglipose capsules in high performance liquid phase: the APIs or preparations containing voglipose and related substances are dissolved in diluents and diluted to a concentration of 10mg / mL; Separation of voglipose and related substances with 1-phenyl-3-methyl-5-pyrazolinone and sodium hydroxide as derivatization reagents, octadecyl bonded porous silica gel as filler, and potassium dihydrogen phosphate buffer-acetonitrile mixed solution as the mobile phase; The dilution is water. The column is a C18 column, the mobile phase is pH 7.0 potassium dihydrogen phosphate buffer - acetonitrile (80:20), the flow rate is 0.7ml / min, the column temperature is 35 °C, the detection wavelength is 245 nm.
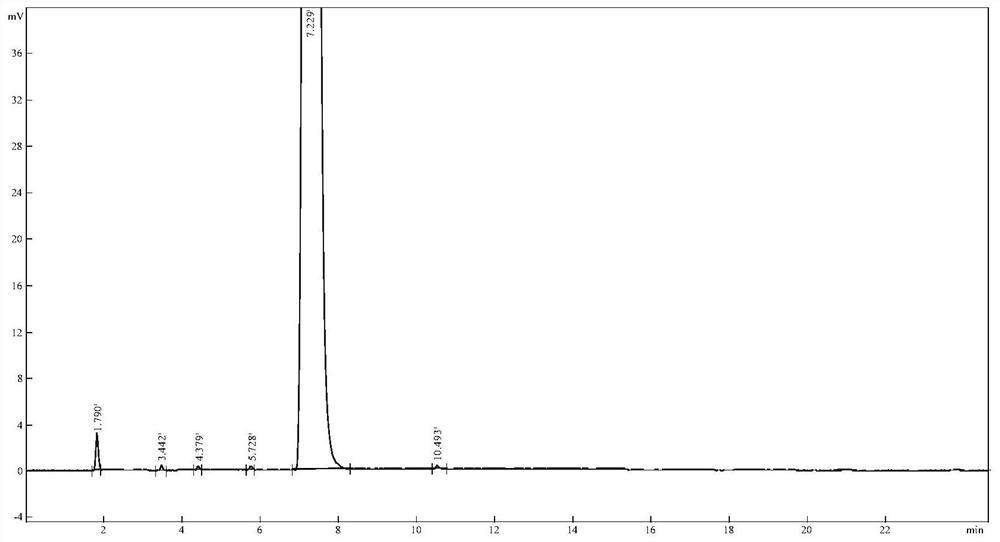
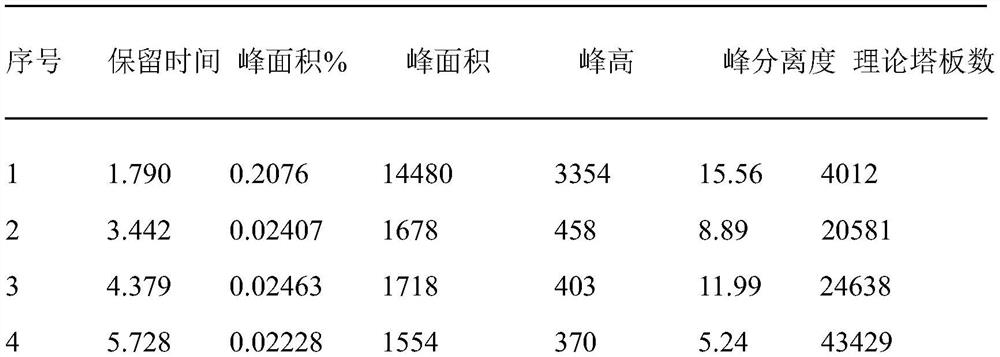
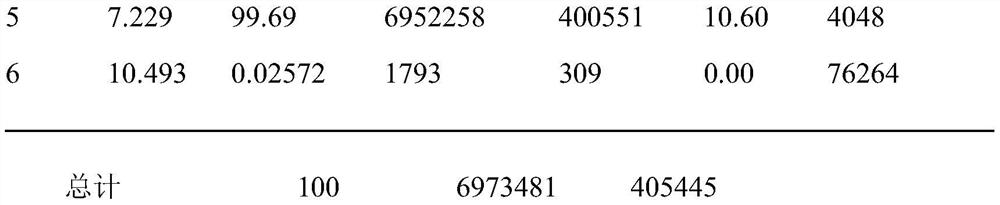
[0020] Chromatographic separation results are attached Figure 1 As shown, the data is shown in Table 1, from the attached Figure 1 It can be seen that the peak retention time of voglipose chromatography is 7.229min, and the se...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com