Voglibose tablet with high dissolution rate and preparation method thereof
A technology for voglibose and tablets, which is applied in the field of voglibose tablets and its preparation, and can solve the problems of increasing the risk of adverse reactions, affecting the curative effect of voglibose, and the types and dosage of excipients. , to increase the dissolution surface area, ensure a high degree of dispersion, and improve the dissolution performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
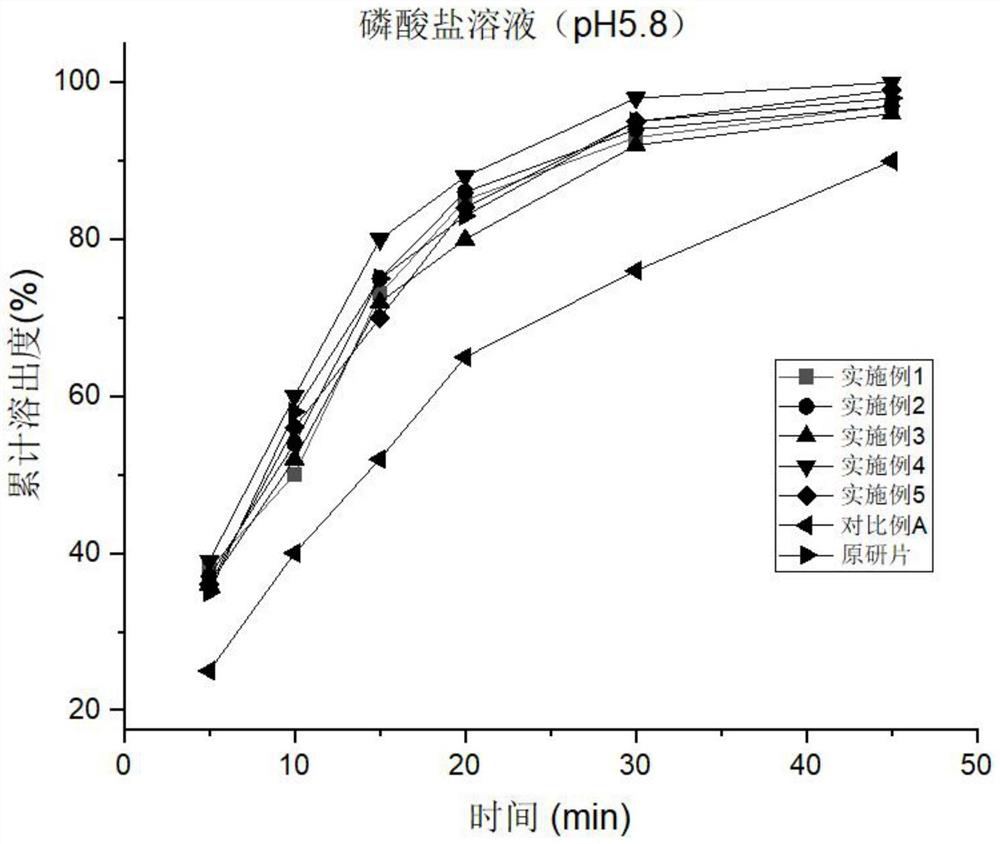
Examples
Embodiment 1
[0022] Example 1: Preparation of Voglibose Tablets
[0023] The voglibose solid dispersion of the present embodiment is composed of voglibose raw materials and carrier materials PEG-4000 and PEG-6000 according to the weight ratio of 1:1:0.5; the formulation of tablet accessories is as follows:
[0024]
[0025] Preparation Process:
[0026] (a) Dissolving: disperse the carrier materials PEG-4000 and PEG-6000 in 42% (V / V) ethanol, ultrasonicate until dissolved, and then add voglibose to obtain a mixed solution;
[0027] (b) Spray drying: spray drying the mixed solution in step (a), and the spray drying process conditions are: the inlet air volume is 40-60 m 3 / h, the inlet air temperature is 50-70°C, the spray speed is 8-10g / min, the atomization pressure is 0.3-0.5MPa, and the outlet air temperature is 45-55°C to obtain voglibose solid dispersion powder;
[0028] (c) preparation of solid preparation: take the solid dispersion of voglibose prepared in step (b), add adjuvant...
Embodiment 2
[0029] Example 2: Preparation of Voglibose Tablets
[0030] The voglibose solid dispersion of this embodiment is composed of voglibose raw materials and carrier materials PEG-4000 and PEG-6000 according to the weight ratio of 1:0.5:0.5; the tablet formulation is composed as follows:
[0031]
[0032] Preparation Process:
[0033] (a) Dissolving: disperse the carrier materials PEG-4000 and PEG-6000 in 60% (V / V) ethanol, ultrasonicate until dissolved, and then add voglibose to obtain a mixed solution;
[0034] (b) Spray drying: spray drying the mixed solution in step (a), and the spray drying process conditions are: the inlet air volume is 40-60 m 3 / h, the inlet air temperature is 50-70°C, the spray speed is 8-10g / min, the atomization pressure is 0.3-0.5MPa, and the outlet air temperature is 45-55°C to obtain voglibose solid dispersion powder;
[0035] (c) preparation of solid preparation: take the solid dispersion of voglibose prepared in step (b), add adjuvant lactose T8...
Embodiment 3
[0036] Example 3: Preparation of Voglibose Tablets
[0037]The voglibose solid dispersion of the present embodiment is composed of the voglibose raw material and the carrier material polyvinyl alcohol MXP according to the weight ratio of 1:2; the composition of the tablet auxiliary material is as follows:
[0038]
[0039] Preparation Process:
[0040] (a) dissolving: disperse the carrier material polyvinyl alcohol MXP in 50% (V / V) ethanol, ultrasonicate until dissolved, and then add voglibose to obtain a mixed solution;
[0041] (b) Spray drying: spray drying the mixed solution in step (a), and the spray drying process conditions are: the inlet air volume is 40-60 m 3 / h, the inlet air temperature is 50-70°C, the spray speed is 8-10g / min, the atomization pressure is 0.3-0.5MPa, and the outlet air temperature is 45-55°C to obtain voglibose solid dispersion powder;
[0042] (d) preparation of solid preparation: take the solid dispersion of voglibose prepared in step (b), a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com