Process for the preparation of voglibose
A technology for voglibose and voglibose is applied in the field of preparing acid addition salts of voglibose and preparing crystalline hydrochloride of voglibose, and can solve the problem of low total product yield, Difficulty in separation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] Example 1: Preparation of tetra-o-benzyl-5-[[2-hydroxyl-1-(hydroxymethyl)ethyl]amino]-1-C-(hydroxymethyl)-1,2,3,4 -Cyclohexanethritol
[0082] Add 2-amino-1,3-propanediol (20.1 g, 220 mmol) to tetra-o-benzyl-5-oxo-1-C-(hydroxymethyl)-1,2,3,4- Cyclohexanethritol (35.0 g, 63.4 mmol) in methanol (350 mL) and stirred for 60 minutes. Then, sodium cyanoborohydride (14 g, 222 mmol) was added to the reaction mixture. Concentrated hydrochloric acid was added to adjust the pH to about 8.0, and the reaction mixture was stirred overnight. The reaction mixture was partitioned between water and ethyl acetate. The ethyl acetate layer was dried over anhydrous sodium sulfate, and concentrated to obtain the title compound as a light yellow syrup.
[0083] Yield: 38.6g
[0084] HPLC purity: 90.0%
[0085] 1 HNMR (CDCl 3 ), δ: 1.60 (1H, dd, J = 2.1, 15Hz), 1.92 (1H, dd, J = 2.7, 15Hz), 2.75 (1H, m), 3.20 (1H, d, J = 8.4Hz), 3.44 (1H, m), 3.50-3.69 (7H, m), 4.10 (1H, m), 4.39 (2H, ...
Embodiment 2
[0086] Embodiment 2: Preparation of voglibose hydrochloride
[0087] Add 5% palladium on carbon (13g) and 4% hydrogen chloride solution (20ml) to tetra-o-benzyl-5-[[2-hydroxyl-1-(hydroxymethyl)ethyl]amino]-1-C- (Hydroxymethyl)-1,2,3,4-cyclohexanetetrol (13.0g, 20.73mmol) in methanol and tetrahydrofuran (1:1, 260ml) solution, room temperature, 3.0-3.5Kg / cm 2 The mixture was hydrogenated with shaking for 3 hours. The solid was removed by filtration and washed with methanol. The combined filtrate and washes were concentrated. Then, ethanol was added to the obtained residue, and the solvent was completely recovered. This process is repeated several times to remove traces of water. Methanol (35 ml) was then added and stirred at room temperature for 1 hour. The product was filtered to obtain voglibose hydrochloride as a white crystalline solid.
[0088] Yield: 5.0g
[0089] 1 HNMR (D 2 O), δ: 1.94 (1H, d, J = 3.0, 16.2Hz), 2.33 (1H, d, J = 2.1, 16.2Hz), 3.60-3.70 (4H, m), 3...
Embodiment 3
[0091] Embodiment 3: Preparation of voglibose
[0092] 20% triethylamine in methanol was added to a suspension of voglibose hydrochloride (5.0 g, 16.47 mmol) in 40 ml of methanol to adjust the pH to about 8.8-9.0. The suspension became clear and then the free base crystallized. Stirring for 1 hour, filtration and washing with methanol afforded voglibose as a white crystalline solid which could be recrystallized from methanol.
[0093] Yield: 3.0g
[0094] HPLC purity: 99.9%
[0095] 1 HNMR (D 2 O), δ: 1.55 (1H, dd, J = 2.1, 15Hz), 2.10 (1H, dd, J = 2.7, 15Hz), 2.9 (1H, m), 3.40-3.55 (2H, m), 3.59 (2H , m), 3.64-3.80 (5H, m) 3.88 (1H, t, J=9.6Hz)
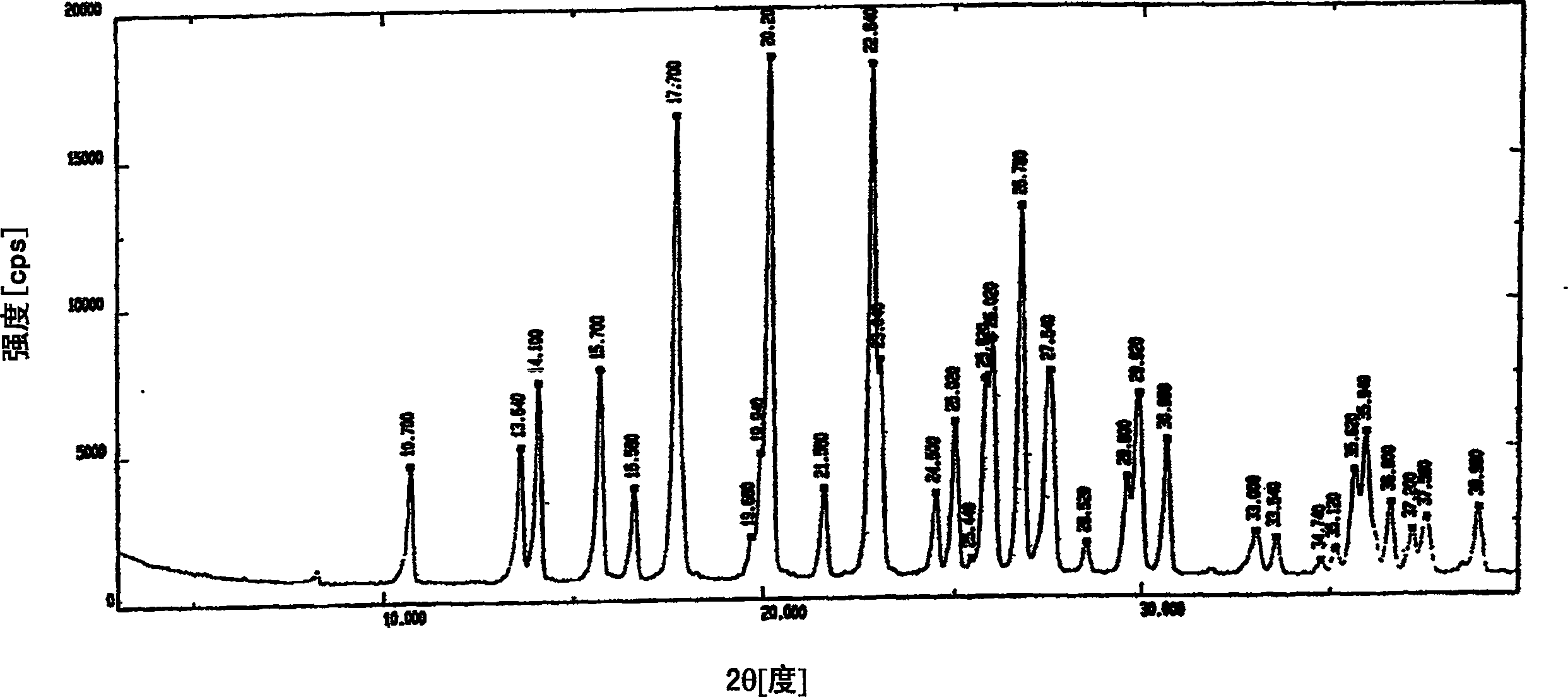
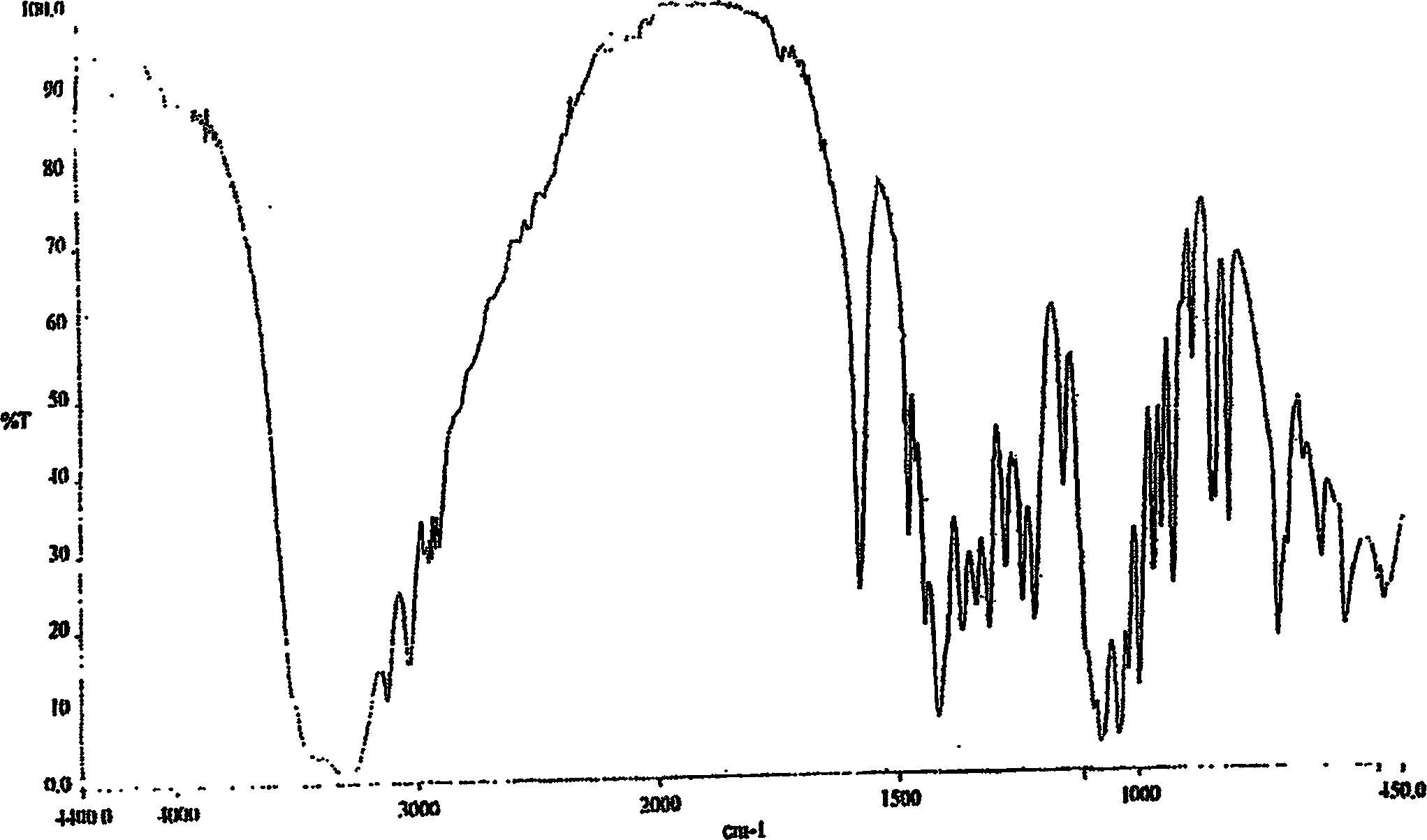
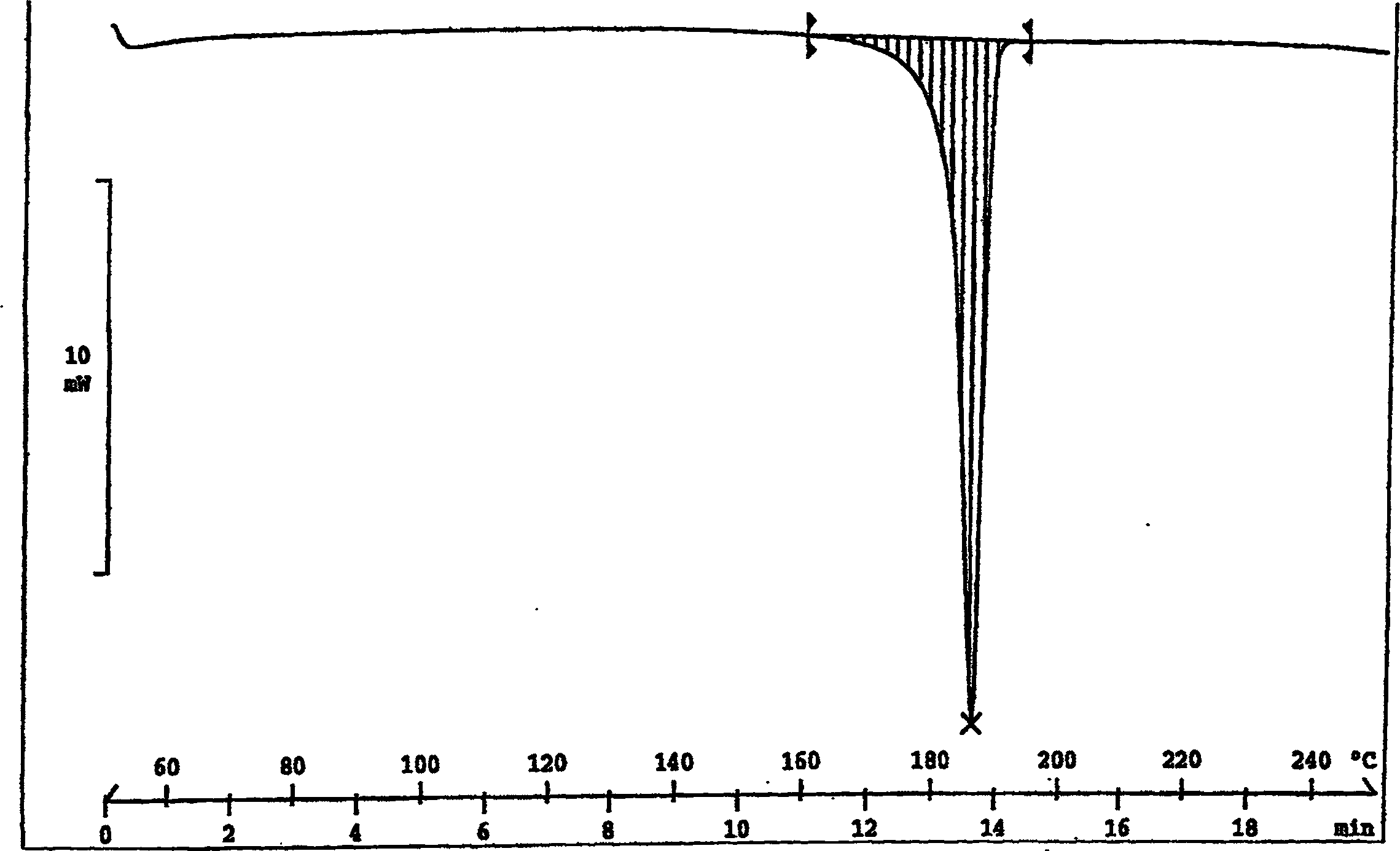
[0096] The XRD spectrum, IR spectrum and DSC pattern are similar to Figures IV, V and VI, respectively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com