Oral preparation for slowing down absorption of alpha-glycosidase inhibitor and enhancing hypoglycemic drug effect
A technology of glycosidase inhibitors and oral preparations, which is applied in the field of application, can solve the problems of not being able to inhibit α-glucosidase, and achieve the effects of increasing the action time, improving drug efficacy, and delaying absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] The formulation and preparation process of embodiment 1 alpha-glucosidase inhibitor oral preparation
[0020] Table 1. Formulations of Oral Formulations of Alpha-Glucosidase Inhibitors
[0021]
[0022] Tablet preparation process in oral formulations of alpha-glucosidase inhibitors (1-12)
[0023] Take by weighing 12.5 grams of α-glucosidase inhibitor dry powder according to the ratio of Table 1 (1-12) and sieve and mix with 62.5 grams of corresponding adhesive auxiliary materials to obtain 75 grams of bulk drug powder; then press bulk drug powder: mannitol=3: 1 Weigh 25 grams of mannitol, sieve and mix with raw drug powder; add an appropriate amount of 95% ethanol as a wetting agent, make a soft material, extrude and sieve to obtain granules, dry the granules, and add 0.3% stearic acid Magnesium, granulated, mixed evenly, adjust the lower punch and upper punch of the tablet press to an appropriate pressure, and tablet. The corresponding α-glucosidase inhibitor tab...
Embodiment 2
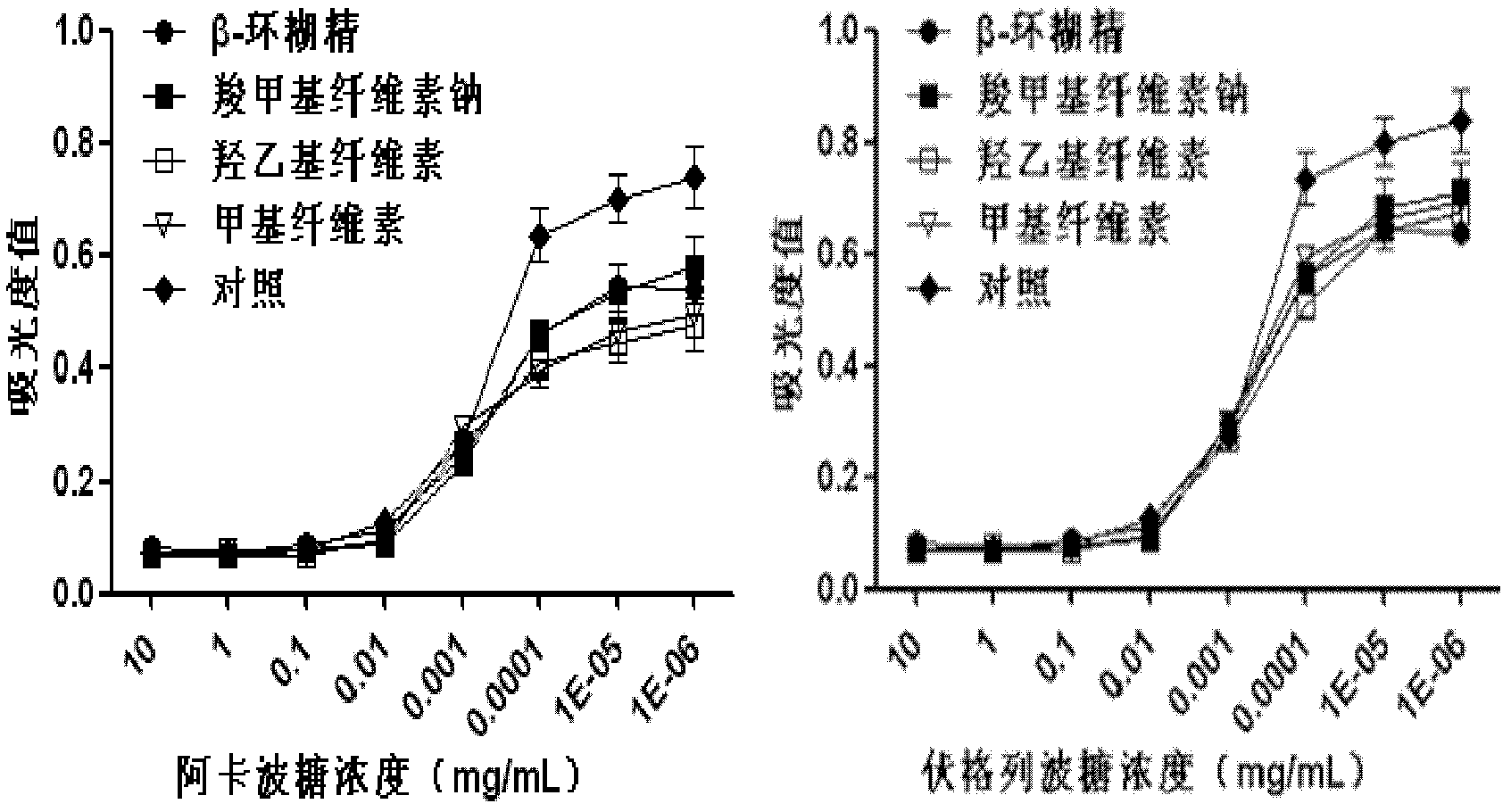
[0046] Example 2. Effect of adjuvant on α-glucosidase inhibitory activity
[0047] Select sodium carboxymethylcellulose, hydroxyethylcellulose, methylcellulose, and β-cyclodextrin respectively as adhesive auxiliary materials, and prepare No. 1-4 acarbose sustained-release preparations in Table 1 and 5- No. 8 voglibose sustained-release preparation, to investigate the effect of adding excipients on the inhibitory activity of α-glucosidase inhibitor acarbose or voglibose. The change in inhibitory activity was measured by detecting the amount of glucose produced by the reaction of α-glucosidase with the substrate starch. Take 1 milliliter of 5% of the above-mentioned various auxiliary materials and add them to the test tube, then add 30 microliters of serial dilutions (10, 1, 10 -1 , 10 -2 , 10 -3 , 10 -4 , 10 -5 mg / ml) acarbose or voglibose solution and 25 μl rat-derived α-glucosidase crude enzyme solution, vortexed and added 1 mg / ml starch solution 100 μl as The substrate...
Embodiment 3
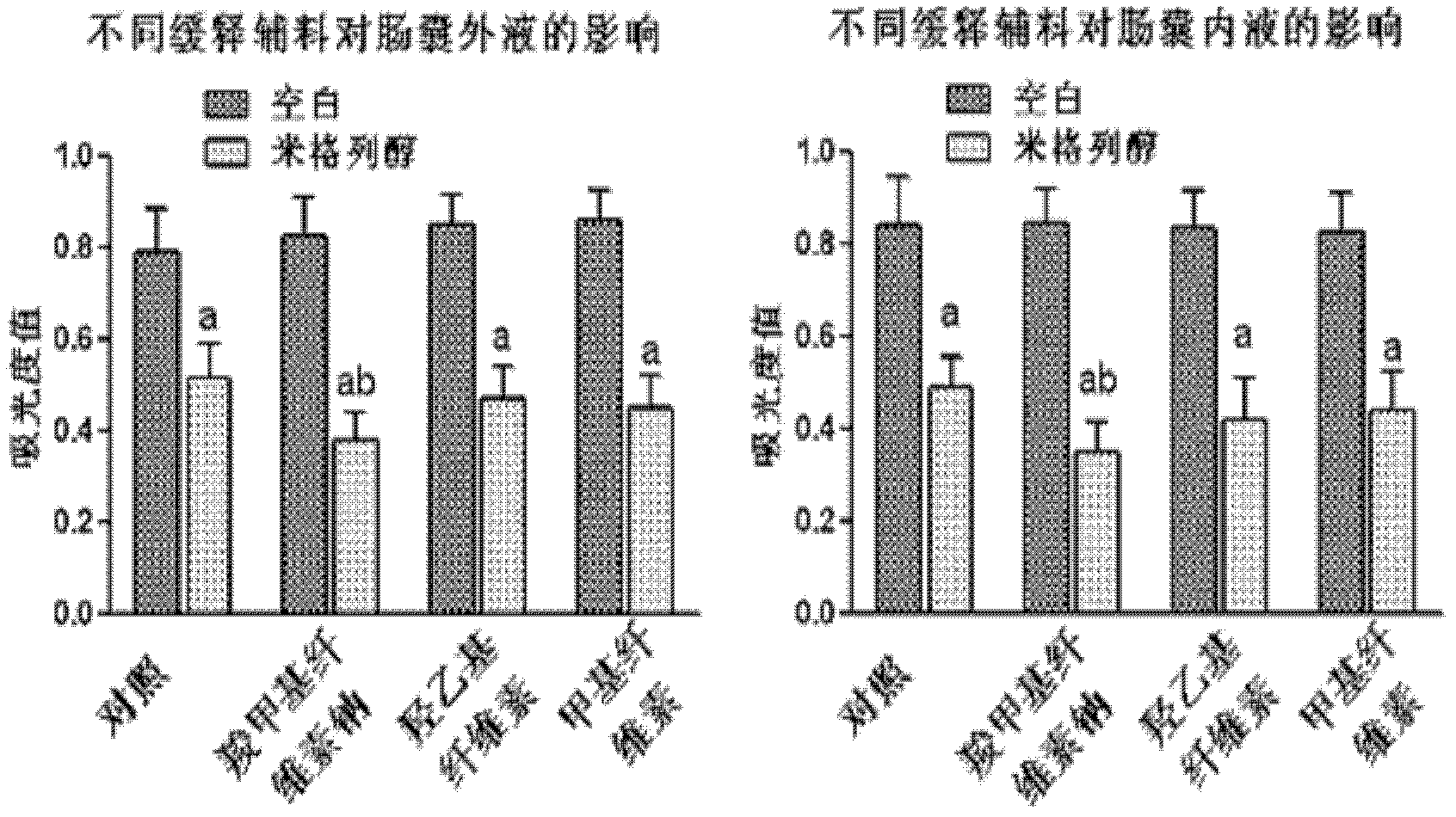
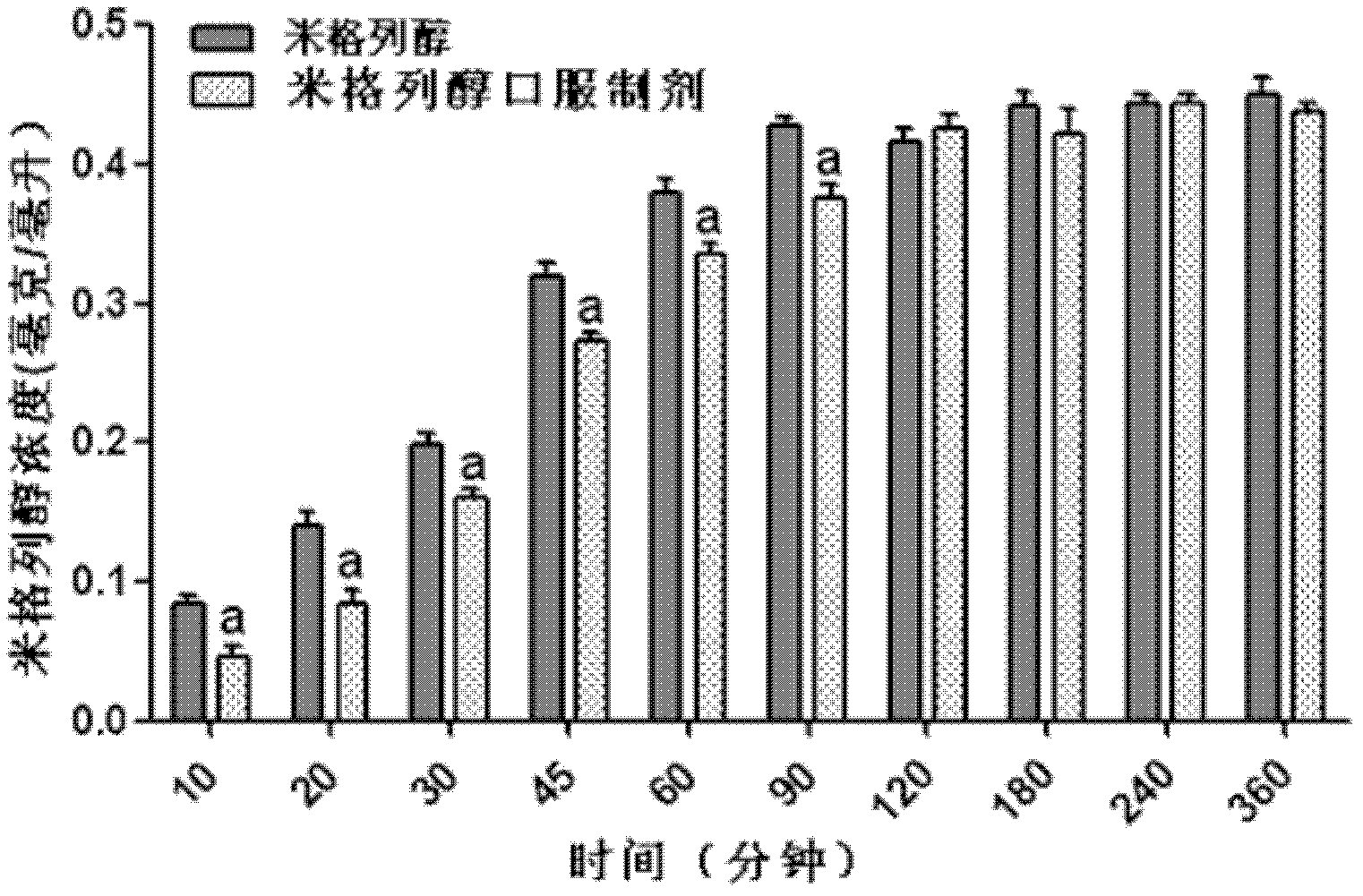
[0048] Example 3. Effects of different excipients on glucose production and transport
[0049] In order to further study the effect of adding adhesion excipients on glucose production and transport, using the inverted intestinal sac experimental model, select male SD rats fasted for 16 hours, kill them and take out the small intestine, cut the small intestine into small pieces of 3.5-4.0 cm, and invert the small intestine. One end of the inverted intestinal sac was ligated with cotton thread, and 200 microliters of Krebs-Henseleit buffer was poured into the intestinal sac from the other end and ligated. The intestinal pouch was filled with 4 ml of 1% starch aqueous solution, and No. 9-12 miglitol sustained-release preparations in Table 1 were added, and the same dose of miglitol solution alone was used as a control. Incubate for 60 minutes at 37°C in a shaking water bath. The reaction was terminated by adding 10 μl of 1 molar hydrochloric acid solution. Collect the liquid i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com