A kind of voglibose tablet and preparation method thereof
A technology for voglibose and sugar tablets, which is applied to pharmaceutical formulations, medical preparations with inactive ingredients, and medical preparations containing active ingredients, etc., can solve the problem of low dissolution and uniform content of voglibose tablets. It can protect the gastric mucosa, take it easily, and increase the absorption points.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
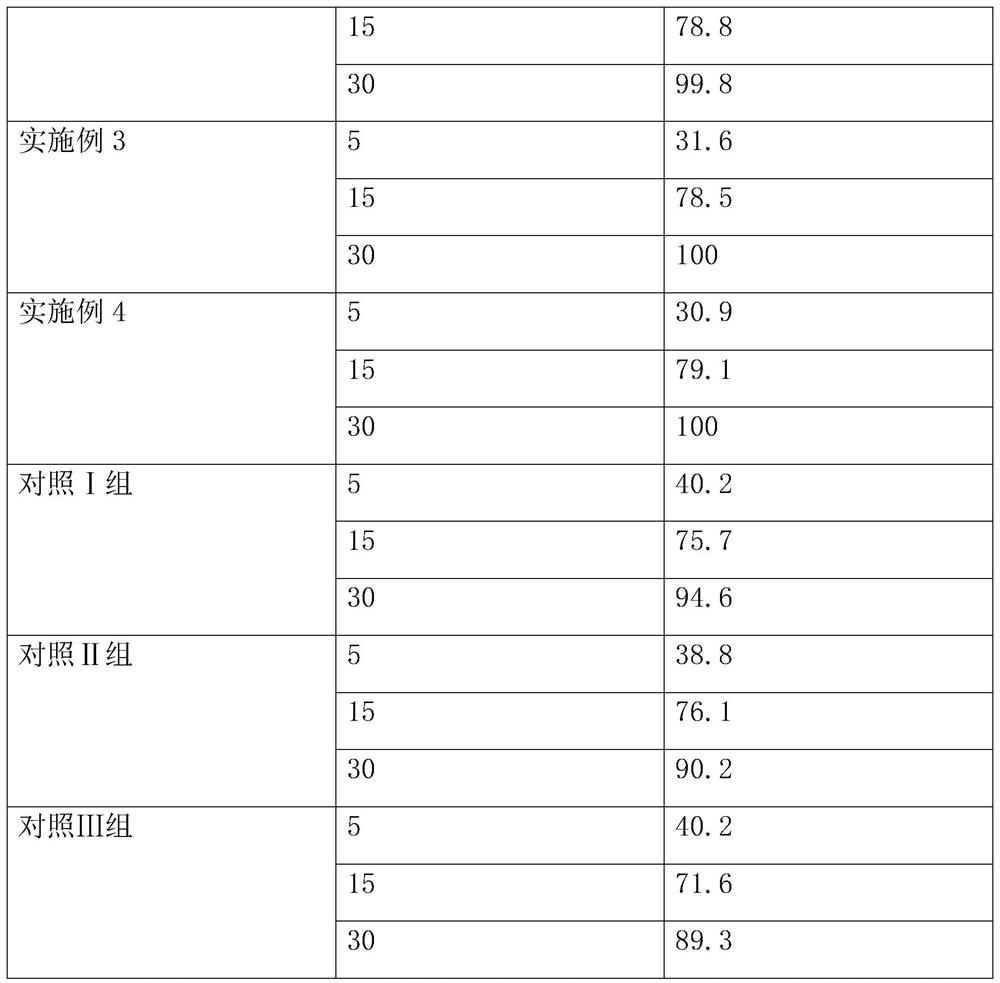
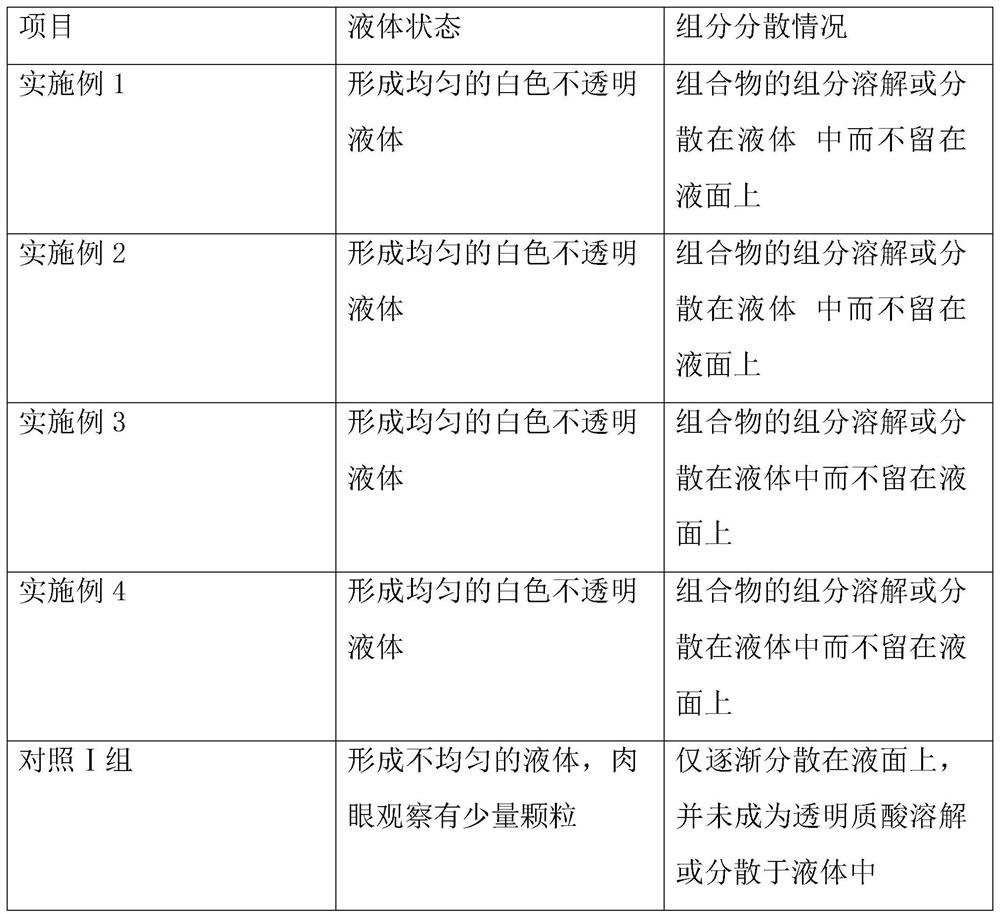
Examples
Embodiment 1
[0024] The voglibose tablet of this embodiment includes the following raw materials: 13 parts of voglibose, 480 parts of lactose, 45 parts of microcrystalline cellulose, 47 parts of hydroxypropyl cellulose, 120 parts of crospovidone, hard 6 parts of magnesium fatty acid, 5 parts of polysorbate 80, 16 parts of micropowdered silica gel, 11 parts of xanthan gum, 28 parts of magnesium carbonate, 16 parts of isopropylene glycol, 17 parts of carboxymethyl cellulose calcium, 17 parts of sodium alginate, hydrogenated 28 parts of phospholipids, 23 parts of vitamin E ester, 10 parts of sodium hydroxide, 9 parts of potassium hydroxide, 7 parts of povidone K30.
[0025] The preparation method of the voglibose tablet of the present embodiment comprises the following steps:
[0026] 1) Premixing: Pass lactose, microcrystalline cellulose, hydroxypropyl cellulose and crospovidone through a 100-mesh sieve respectively, weigh lactose, microcrystalline cellulose, hydroxypropyl cellulose and cros...
Embodiment 2
[0032] The voglibose tablet of this embodiment includes the following raw materials: 11 parts of voglibose, 475 parts of lactose, 50 parts of microcrystalline cellulose, 50 parts of hydroxypropyl cellulose, 150 parts of crospovidone, hard 7 parts of magnesium fatty acid, 10 parts of polysorbate 80, 7 parts of micronized silica gel, 20 parts of fucus, 27 parts of magnesium carbonate, 20 parts of isopropylene glycol, 15 parts of carboxymethyl cellulose calcium, 14 parts of sodium alginate , 30 parts of hydrogenated phospholipids, 25 parts of vitamin E ester, 13 parts of sodium hydroxide, 10 parts of potassium hydroxide, 6 parts of povidone K30.
[0033] The preparation method of the voglibose tablet of the present embodiment comprises the following steps:
[0034] 1) Premixing: Pass lactose, microcrystalline cellulose, hydroxypropyl cellulose and crospovidone through a 100-mesh sieve respectively, weigh lactose, microcrystalline cellulose, hydroxypropyl cellulose and crospovidon...
Embodiment 3
[0040] The voglibose tablet of this embodiment includes the following raw materials: 10 parts of voglibose, 470 parts of lactose, 49 parts of microcrystalline cellulose, 45 parts of hydroxypropyl cellulose, 130 parts of crospovidone, hard 6 parts of magnesium fatty acid, 10 parts of polysorbate 80, 10 parts of micropowdered silica gel, 15 parts of xanthan gum and fucus, 23 parts of magnesium carbonate, 17 parts of isopropylene glycol, 19 parts of carboxymethylcellulose calcium, 10 parts of sodium alginate, 25 parts of hydrogenated phospholipids, 22 parts of vitamin E ester, 11 parts of sodium hydroxide, 7 parts of potassium hydroxide, and 5 parts of povidone K30.
[0041] The preparation method of the voglibose tablet of the present embodiment comprises the following steps:
[0042] 1) Premixing: Pass lactose, microcrystalline cellulose, hydroxypropyl cellulose and crospovidone through a 100-mesh sieve respectively, weigh lactose, microcrystalline cellulose, hydroxypropyl cell...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com