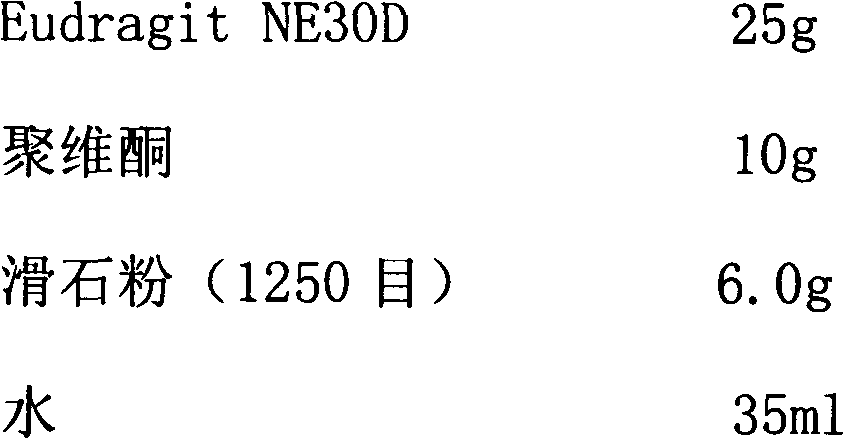Patents
Literature
281 results about "Stomach mucosa" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Health-care flour composition and food
ActiveCN103651657AImprove gastritisSymptoms improvedDough treatmentBakery productsNutritionDuodenal ulcer
The invention provides a health-care flour composition and food and a preparing method thereof. According to the preparing method, hericium erinaceus and flour are used as main raw materials, swelling agents, sweetening agents, oil, salt and the like are added and mixed with water, and then rolling, forming, baking, oil injection and cooling are carried out to obtain various foods made of flour or pastries such as cookies, cakes and noodles. The health-care flour composition and food are even in texture, capable of being stored for a long time easily, good in mouthfeel and rich in nutrient, and market vacancy is filled. The health-care flour composition and food have definite effects in repairing gastric mucosal damage caused by chronic gastritis, or gastric ulcers or duodenal ulcers, relieving abdominal pains caused by chronic gastritis, or gastric ulcers or duodenal ulcers, inhibiting helicobacter pylori, and assisting the treatment of chronic gastritis, gastric ulcers and duodenal ulcers.
Owner:江中食疗科技有限公司
Enzyme linked immunosorbent assay kit for combined diagnosis of gastrosis or evaluation of gastric cancer risks
InactiveCN102087279AIncreased sensitivityImprove featuresComponent separationTissue cultureAntigenPepsinogen I
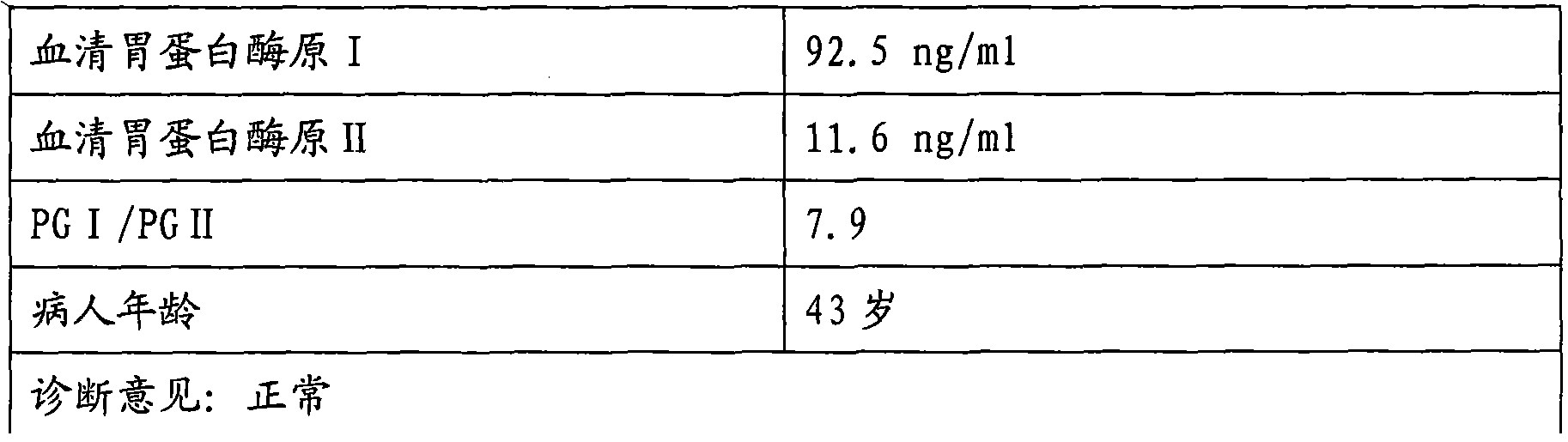
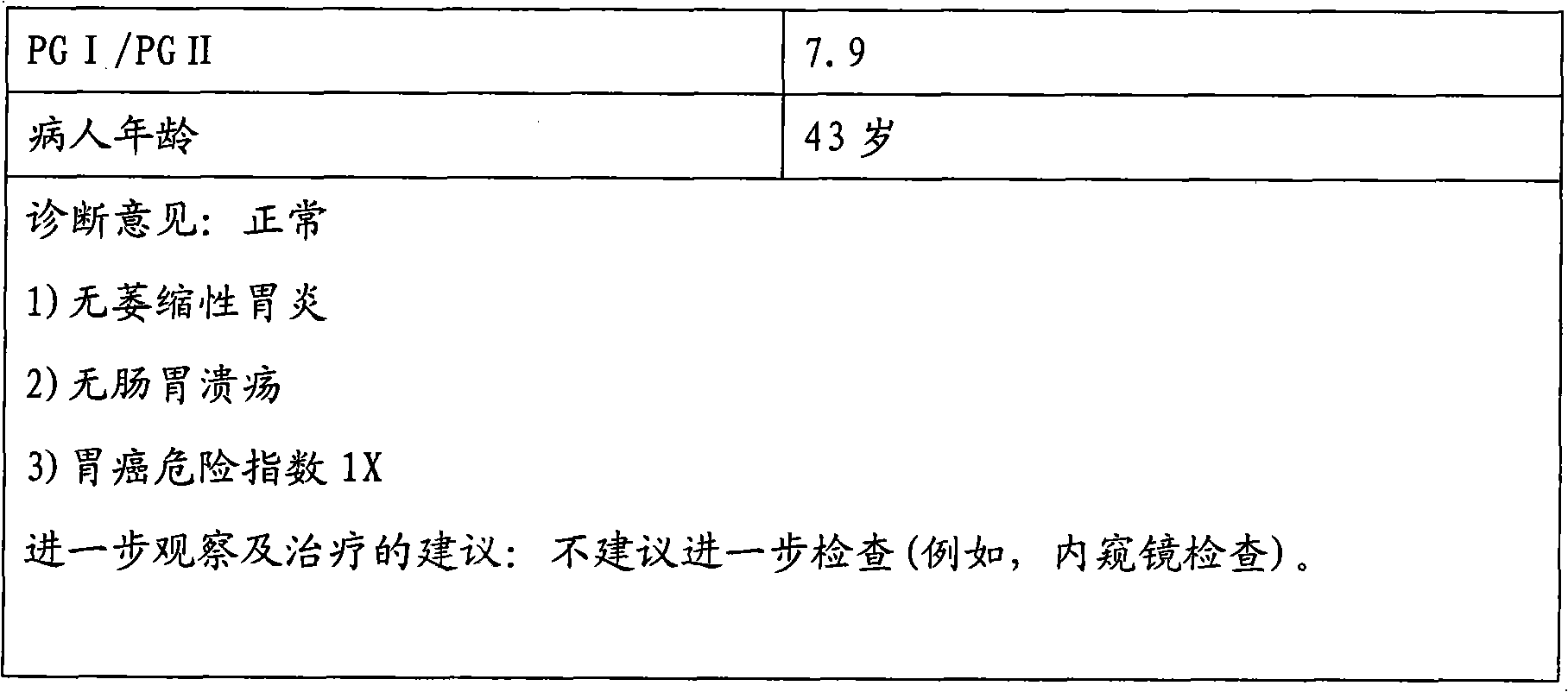
The invention discloses an enzyme linked immunosorbent assay kit for combined diagnosis of the gastrosis or evaluation of gastric cancer risks and a preparation method thereof. The kit comprises a micropore plate coated with an antibody against a pepsin antigen I or an antibody against a pepsin antigen II, an enzyme labeled antibody, a color-developing agent, a stop solution and a concentrated cleaning solution, wherein the pepsin antigen I or the pepsin antigen II is a natural protein obtained from extraction of human gastric mucosa tissue. The kit disclosed by the invention adopts a mouse immunized with pepsinogen I and pepsinogen II which are separated from human gastric mucosa to prepare immunogen of a monoclonal antibody, the used standard sample also adopts the pepsin antigen I or the pepsin antigen II separated from the human gastric mucosa, thereby the defects caused by adopting different structures of animal pepsinogen and human pepsinogen are filled. The kit can be used for accurately diagnosing the gastrosis or early gastric cancer and has the advantages of high sensitivity, strong specificity, good accuracy and the like.
Owner:BEIJING MOKOBIO LIFE SCI CO LTD
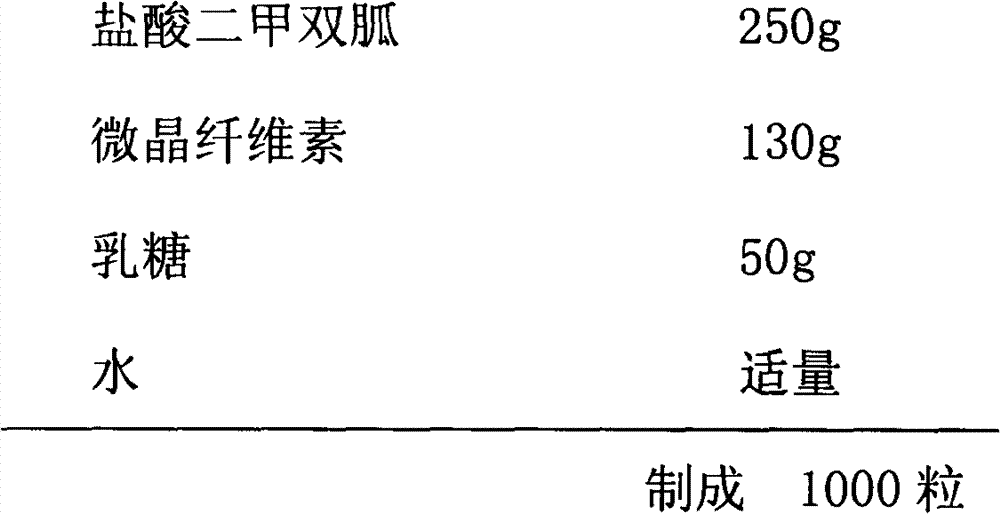
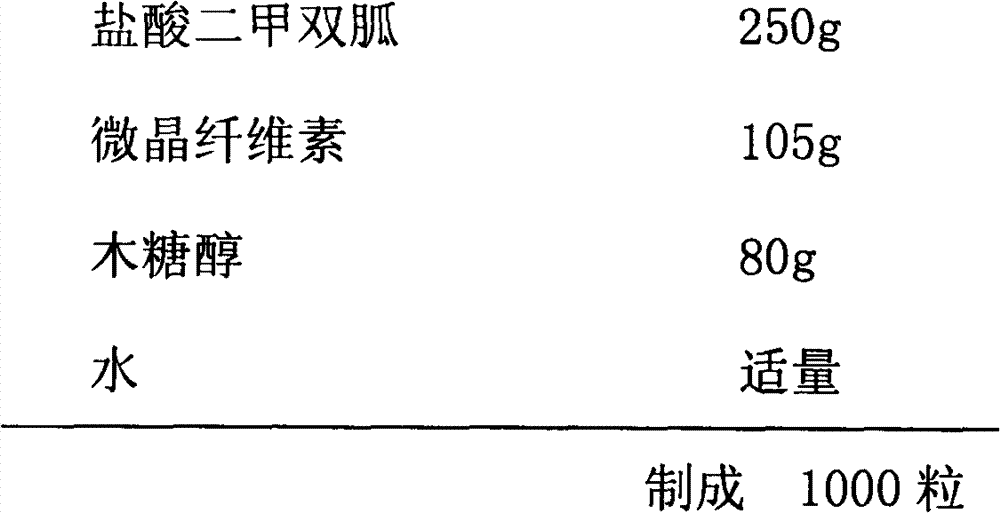
Metformin hydrochloride enteric-coated sustained release tablet and preparation method thereof
ActiveCN101785763AOrganic active ingredientsMetabolism disorderPatient complianceSustained-Release Preparations
The invention discloses a metformin hydrochloride enteric-coated sustained release tablet which is prepared by enteric coating the metformin hydrochloride sustained release tablet. Compared with the prior art, the sustained release tablet integrates with the enteric coating technology to prepare a new form of the metformin hydrochloride enteric-coated sustained release tablet. By using the enteric coating technology, the metformin hydrochloride does not disintegrate in the stomach or stimulate the gastric mucosa, and the adverse reaction of nausea, stomachache and diarrhea caused by medicine taking can be avoided; meanwhile, the metformin hydrochloride is prevented from being damaged by gastric juice, and the bioavailability is improved. The product is a sustained release preparation, the medicine can stably release in vivo, the effective blood concentration can be maintained for a long time, the toxic and side effects caused by over-high blood concentration in a short time are avoided, the medicine taking frequency is decreased, and the patient compliance is improved as well.
Owner:贵州天安药业股份有限公司
Combination tablet with chewable outer layer
ActiveUS20100166810A1Diminishing prostaglandin-mediated flush side effectIncrease in IR levelBiocideAnimal repellantsOral mucosaDosing regimen
A pharmaceutical composition in the form of a combination tablet is described. The tablet has a rapidly absorbed component that enters the circulation by traversing the buccal mucosa, oral mucosa and combinations thereof, and a more slowly absorbed component that is swallowed. The therapeutic agent in the swallowed portion is absorbed across the gastric mucosa. The combination tablet may be modified, by varying the specific combinations of excipients, fillers, and the like to effect distinct release rates. In addition, the rapid and slow components may have identical or different therapeutic agents depending on the application to a specific medical condition. One embodiment of the combination tablet includes a prostaglandin inhibitor in the rapidly absorbed component in order to mitigate the side effects of immediate release niacin that is in the slow absorbing component. Such combination compositions will increase patient compliance with various dosing regimens due to the resultant decrease in the number of tablets that a patient would need to take on a daily basis.
Owner:VITALS
Aloe bird nest drink food and preparation method thereof
InactiveCN101574165ARefreshing and smooth tasteInvigorate the spleen and moisten the lungsFood preparationFood technologyBird nest
The invention belongs to the technical field of drink foods, and in particular relates to an aloe bird nest drink food and a preparation method thereof. The aloe bird nest drink food comprises the following raw materials e by weight percentage: 0.05 to 40 percent of aloe, 0.005 to 0.2 percent of bird nest (in terms of dry basis), 1 to 50 percent of edulcorant, 0 to 20 percent of fruit and vegetable juice, 0 to 0.5 percent of acid taste additive and acid taste regulator, 0 to 6 percent of gelata, and 40 to 85 percent of water. The aloe bird nest drink food integrates the efficacy characteristics of the aloe and the bird nest, not only has fresh and lubricous mouthfeel, but also has the health care functions of invigorating the spleen and moistening the lung, protecting gastric mucosa, resisting inflammation and sterilizing, strengthening the immune system, resisting radiation, hairdressing and the like.
Owner:东莞市德亨饮料食品有限公司
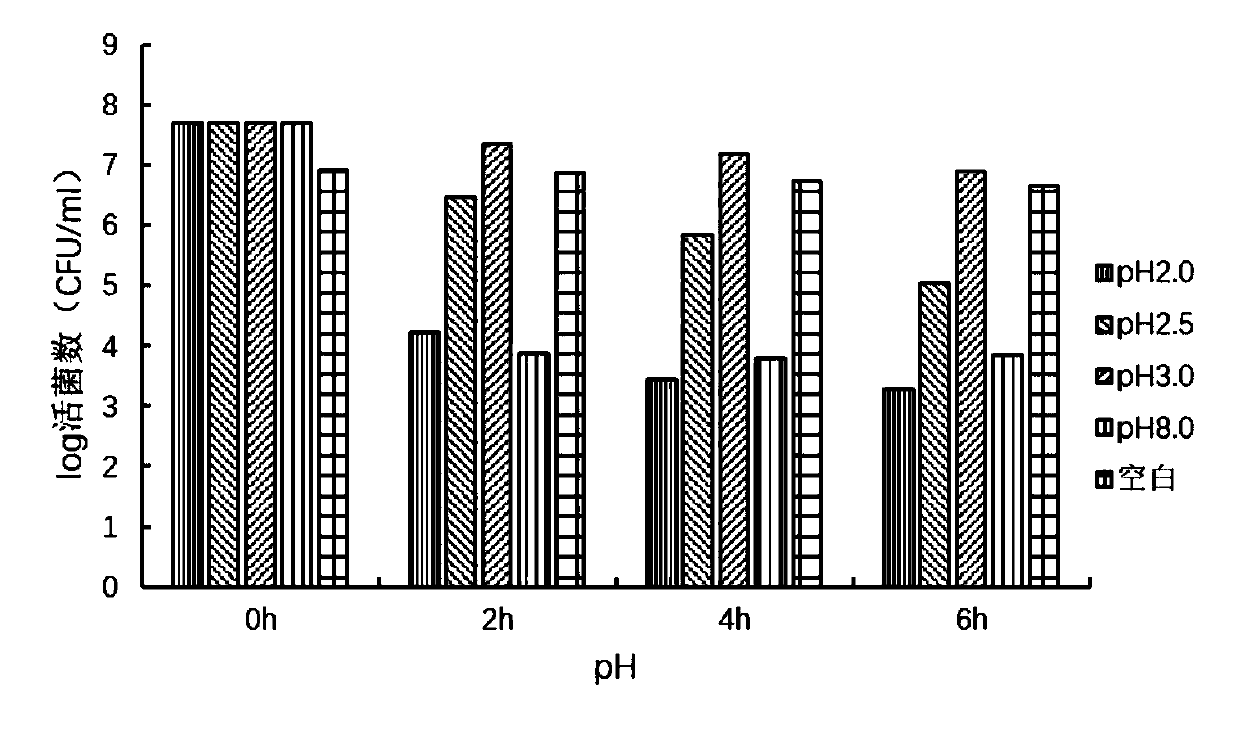
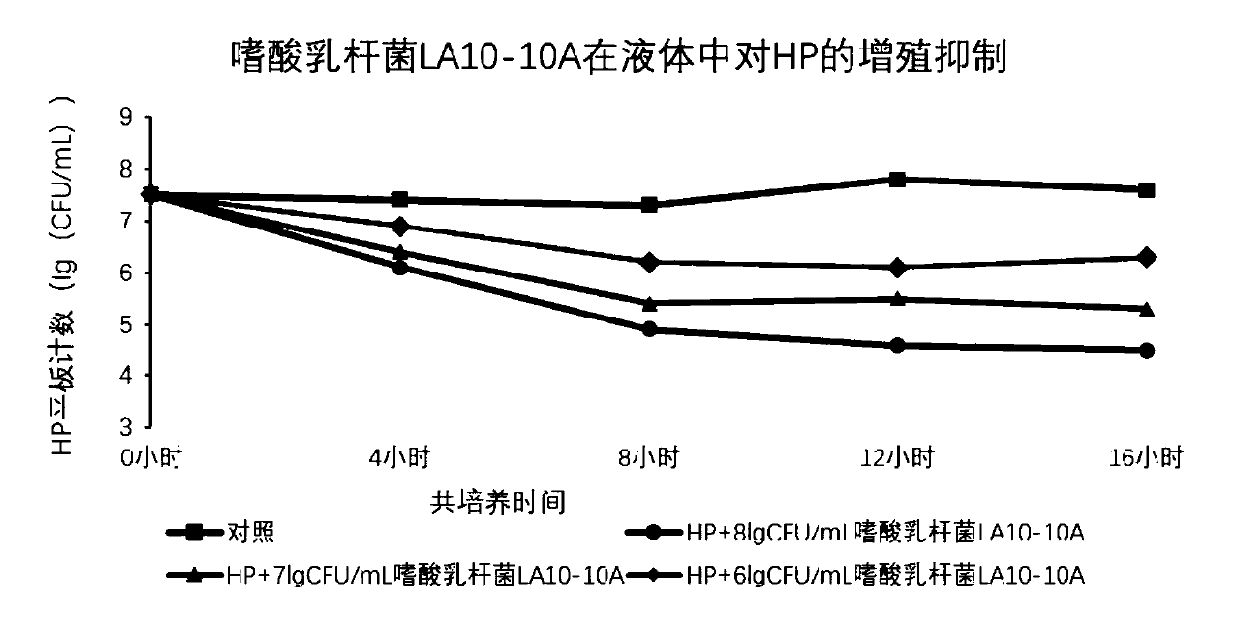
Lactobacillus acidophilus LA-10A capable of inhibiting helicobacter pylori and application thereof
ActiveCN111118106AReduce or prevent infectionInhibit or eliminate colonizationAntibacterial agentsMilk preparationBiotechnologyMetabolite
The invention provides a method of screening a bacterial strain capable of inhibiting helicobacter pylori. The method includes a way that qualitative and quantitative assessment can be directly conducted on screened bacterial strains if the screened bacterial strains can inhibit or eliminate the colonization of the helicobacter pylori on gastric mucosal cells by utilizing DNA fluorescent labels and flow cytometry. According to the invention, the following points are considered when conducting screening model tests in vitro: (1) viability in an extremely acidic stomach environment; (2) inhibition ability of the bacterial strains itself or metabolites of the bacterial strains to HP reproduction in vitro; and (3) ability of inhibiting or relieving the colonization of the helicobacter pylori strains on gastric mucosa. According to the invention, developing the method of directly assessing in vitro if the HP colonization can be inhibited is of great significance to the practical applicationof the bacterial strain provided by the invention. According to the invention, lactobacillus acidophilus LA-10A capable of inhibiting the helicobacter pylori is screened out. According to a test, thelactobacillus acidophilus LA-10A can relieve or prevent the helicobacter pylori infection. The lactobacillus acidophilus LA-10A can be used to prepare functional food or pharmaceutical compositions.
Owner:THANKCOME BIOLOGICAL SCI & TECH CO LTD
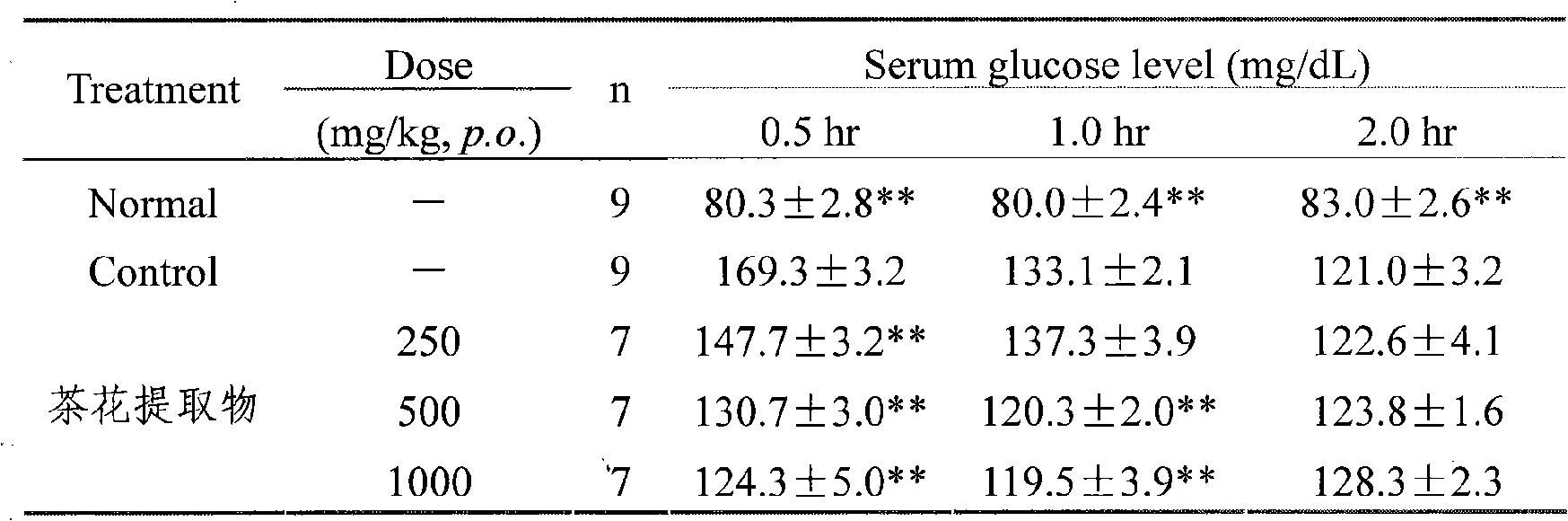
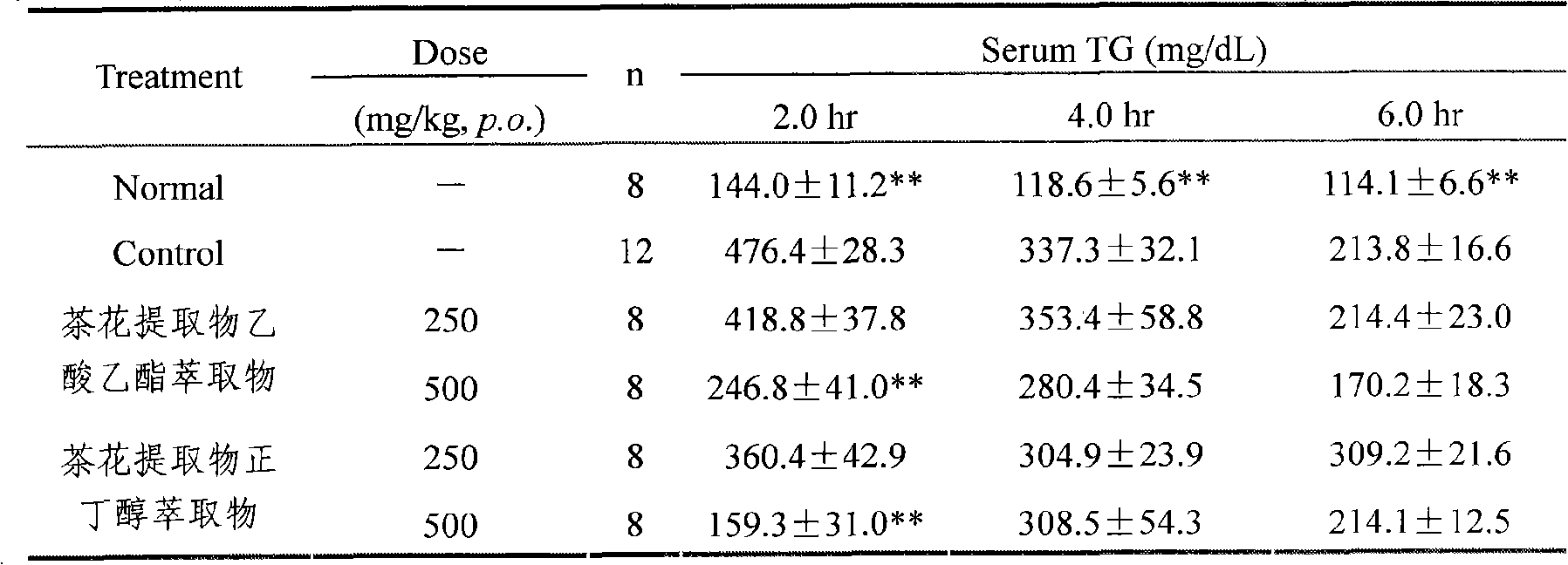
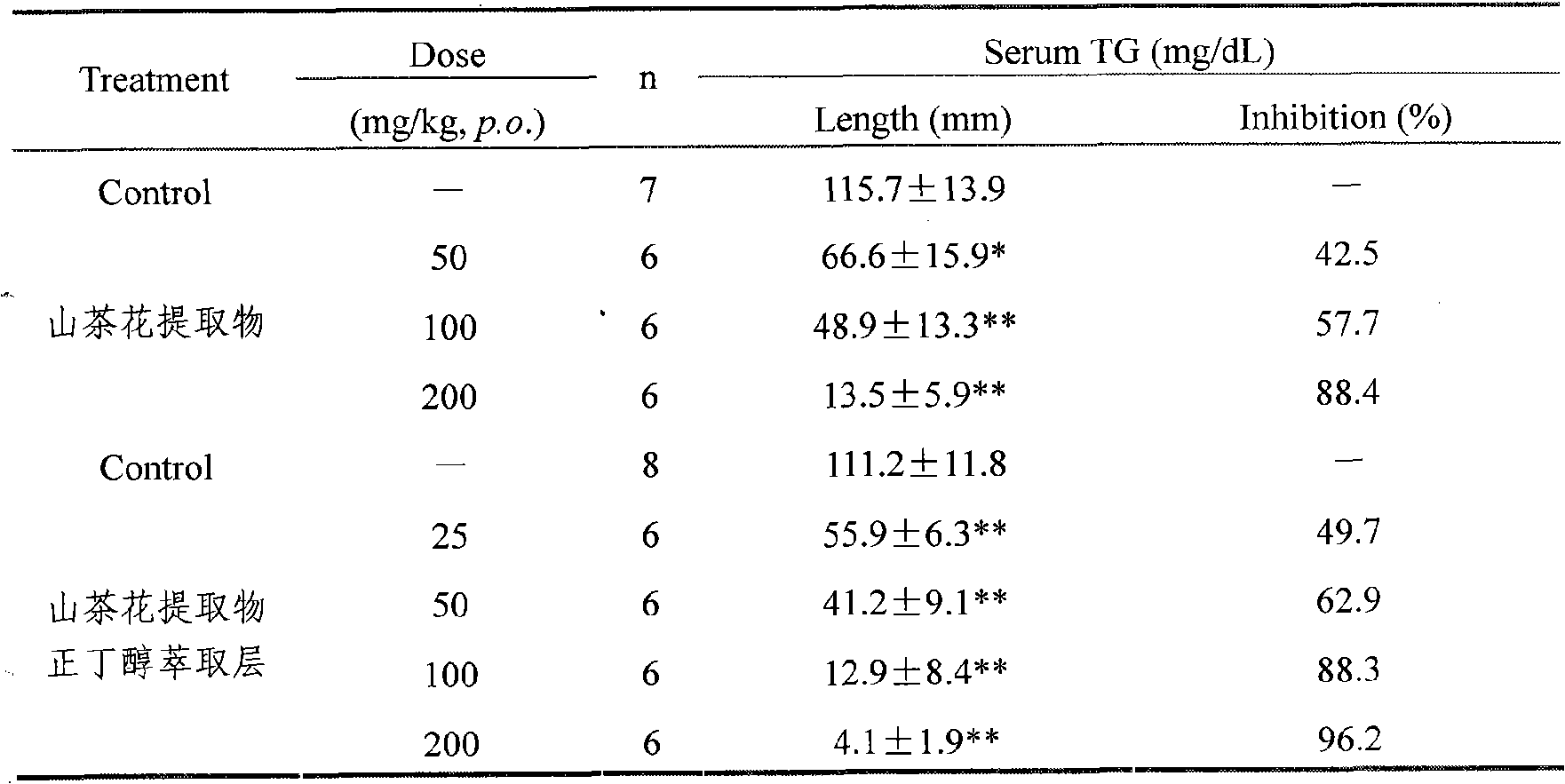
Camellia effective ingredients and extracting method and use thereof
InactiveCN101301401AClear compositionMulti-target synergy Toxic and side effectsMetabolism disorderDigestive systemChemistryCamellia sinensis
The invention provides an extracting method, comprising a water extraction method, an alcohol extraction, a macroporous resin purification method and a solvent extraction method. By adopting the method, the contents of main effective constituents of the obtained extractive are: 1 to 25 percent of TFA, 1 to 20 percent of total Saponins and 1 to 3 percent of phenethyl alcohol glycoside. The invention also provides a method for making the appropriate preparation formulation by using the camellia directly or mixing the solid or liquid of the preparation and the like pharmaceutically in general. The effective constituents of the camellia and the preparation thereof have the functions of reducing blood sugar and lipid and protecting the gastric mucosa, etc. The extracting method has simple implementation and low cost, extracts sufficiently and is suitable for the industrial production; and the extractive has evident activity, precise healing effect and does not have the toxic and side effect.
Owner:SHENYANG PHARMA UNIVERSITY
Dendrobium officinale and hericium erinaceus compound chewable tablet and preparation method thereof
ActiveCN103463457ASolve the problem that the drug effect can not fully workFully absorbedAntibacterial agentsAntipyreticCelluloseMethyl cellulose
The invention discloses a dendrobium officinale and hericium erinaceus compound chewable tablet. According to a formula, the dendrobium officinale and hericium erinaceus compound chewable tablet comprises 2 to 10 parts of dendrobium officinale, 10 to 30 parts of hericium erinaceus, 10 to 70 parts of Guangxi dioscorea opposita thunb, 3 to 5 parts of corrective agent, 20 to 30 parts of hydroxypropyl methyl cellulose, 5 to 15 parts of maltodextrin, and 1 to 3 parts of lubricant. The preparation method comprises the following steps: respectively crushing all the raw materials; screening with 500 meshes to obtain submicron powder; combining in proportion as indicated in the formula; uniformly agitating; adding ethyl alcohol to granulate in a wet manner; drying at a temperature of 60 to 80 DEG C until the moisture content is up to 4 to 5% in percentage by weight; cooling to reach room temperature; screening with 60 meshes; adding the lubricant to mix; then performing to obtain the dendrobium officinale and hericium erinaceus compound chewable tablet. The chewable tablet has the health care effects of clearing heat and promoting fluid, protecting gastric mucosa, nourishing both stomach and liver, diminishing inflammation and relieving pain, replenishing Qi and tonifying lung, and regulating lung and reducing phlegm.
Owner:AGRI PROD PROCESSING INST GUANGXI ACADEMY OF AGRI SCI +1
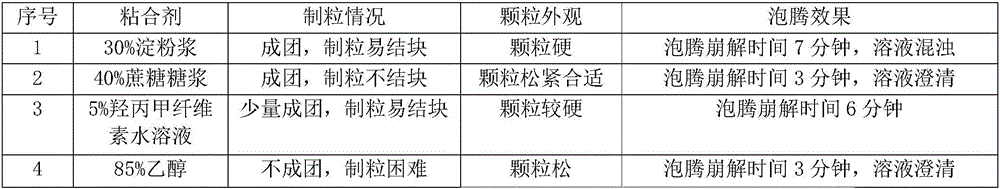
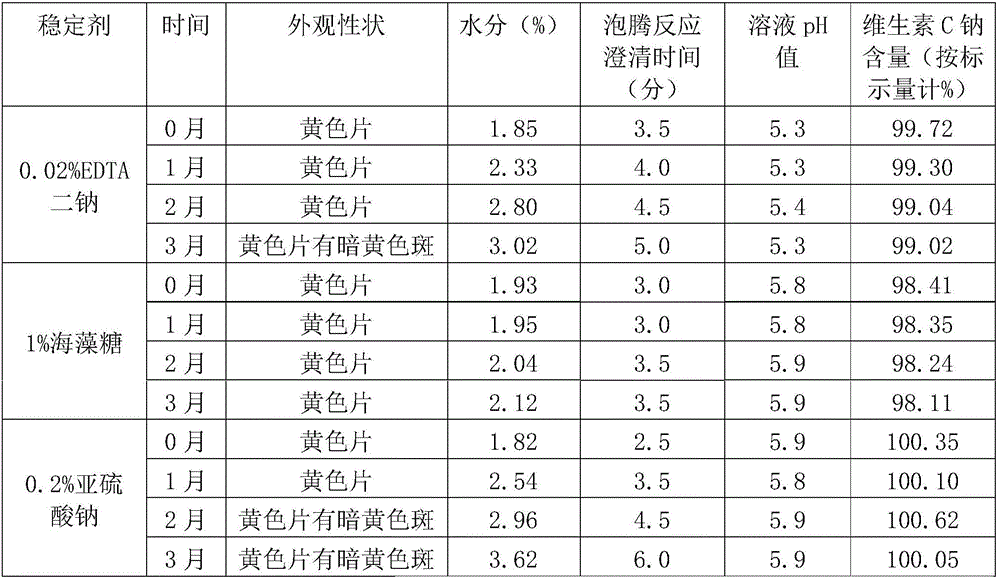
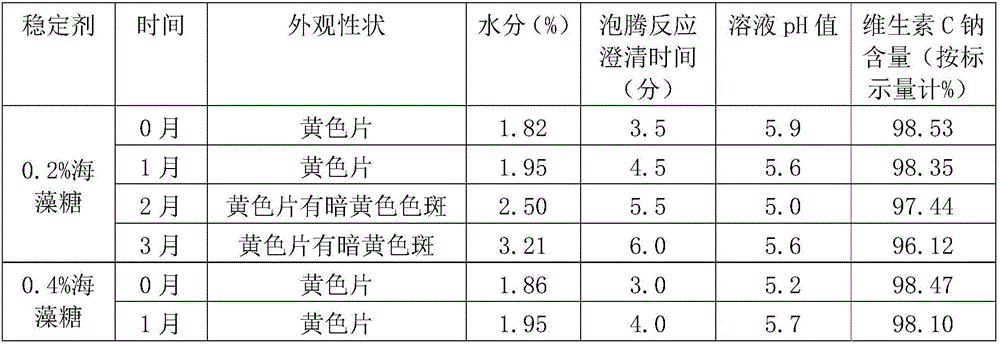
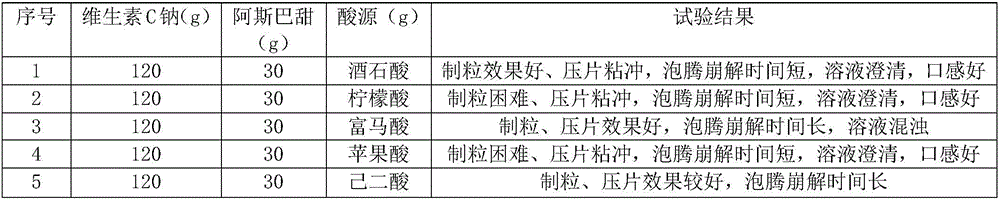
Effervescent tablet containing vitamin C sodium and preparation method of effervescent tablet
InactiveCN106420655ASuitable for long-term useTake applicableOrganic active ingredientsNervous disorderSodium bicarbonateEffervescent tablet
The invention discloses an effervescent tablet containing vitamin C sodium. The effervescent tablet is prepared from the following components in parts by weight: 200-1120 parts of the vitamin C sodium, 560-1300 parts of tartaric acid, 600-1500 parts of sodium bicarbonate and 200-600 parts of sucrose. The effervescent tablet containing the vitamin C sodium provided by the invention aims at solving the problems of an existing vitamin C effervescent tablet which is high in acidity after being dissolved, relatively serious in irritation to oral cavities, throats and esophagi as well as to gastric mucosae and not applicable to long-term taking, the problems that in a storage process, vitamin C can become oxidized and invalid easily and the effervescent tablet is easily affected with damp and easily absorb moisture, and the like; and the preparation (the effervescent tablet) is simple and convenient and is stable in quality.
Owner:GUANGXI SANPOTEL HEALTH IND CO LTD
Medicine composition for treating stomach disease, its preparation method and use
ActiveCN1579473AFast treatmentSymptom Pain Relief QuicklyDigestive systemUnknown materialsSodium bicarbonateAdditive ingredient
The invention discloses a medicine compound for curing tummy bug, which including following ingredients and proportion: bismuth aluminic acid 100-300 shares, heavy magnesium carbonate 100-600 shares, sodium bicarbonate 100-400 shares, liquorice extractive powder 100-500 shares, frank mouse lippich 1-50 shares, fennel powder 1-50 shares, aloe powder 1-50 shares or / and grassleaved sweetflag rhizome 1-50 shares. It also discloses the manufacturing method for the medicine, the compound medicine is one compound preparation resisting acid and protecting gastric mucosa, it can be applied to cure stomach and duodenum abscess, chronic superficial gastritis, overmuch stomach acid, and nerve indigestion and so on.
Owner:HONGMEI PHARMA CHINA
Weibimei medicical composition for treating stomach disease and its preparation method
ActiveCN1824131AFast treatmentSymptom Pain Relief QuicklyDigestive systemAluminium/calcium/magnesium active ingredientsSodium bicarbonateBismuth aluminate
A composite medicine for neutralizing gastric acid, protecting gastric mucosa, treating gastrospasm, promoting appetite, etc is prepared from the extracts of aloe and liquorice root, the volatile oil of fennel and grass-leaved sweetflag rhizome, bismuth aluminate, heavy magnesium carbonate, sodium bicarbonate and davurian buckthorn bark through mixing and adding the pharmacologically acceptable auxiliary.
Owner:HONGMEI PHARMA CHINA
Composition and preparation method thereof
InactiveCN107518383AReduce ulcer indexUlcer Index InhibitionDigestive systemBird material medical ingredientsDuodenal ulcerSpleen
The invention provides a composition. The composition is characterized by being prepared from the following ingredients in parts by weight: ginseng roots 1-15, poria cocos 3-25, Chinese yam 5-50, lotus seeds 2-25, coix seeds 3-50, white hyacinth beans 3-25, polished round-grained rice 20-400 or / and oats 20-400. The invention also relates to the application of the composition in health care food or medicines or products having functions of preventing and treating gastric mucosal damage caused by chronic gastritis, gastric ulcer or duodenal ulcer, relieving abdominal pain caused by the chronic gastritis or peptic ulcer and preventing and treating the syndrome of weakness of the spleen and the stomach.
Owner:江中食疗科技有限公司
Metformin hydrochloride sustained-release capsule and its preparation method
InactiveCN103239424AEasy to swallowAvoid disintegrationOrganic active ingredientsMetabolism disorderSide effectPatient compliance
The invention relates to a metformin hydrochloride sustained-release capsule and its preparation method. The metformin hydrochloride sustained-release capsule is prepared by steps of coating a metformin hydrochloride granule sustained-release material and putting into an enteric capsule. In comparison with the prior art, sustained-release granules and enteric capsule filling technology are combined together to prepare the new dosage form of metformin hydrochloride sustained-release (enteric-coated) capsule. As the sustained-release granule coating and enteric capsule filing technology is adopted, metformin hydrochloride will not be disintegrated and will not stimulates gastric mucosa, and adverse reactions such as nausea, stomachache, diarrhoea and the like caused by medication can be avoided. Meanwhile, metformin hydrochloride will not be damaged by gastric juice, and bioavailability of metformin hydrochloride is raised. In addition, the product provided by the invention is also a sustained-release enteric-coated preparation. The medicine can be stably released in a body, effective plasma concentration is maintained for a long time, and toxic and side effect which might be caused by higher plasma concentration within a short time are avoided. The frequency for taking the medicine is reduced, and patient compliance is also raised.
Owner:BOSEN BIO PHARMA SHANXI PROVINCE
Method for producing pumpkin pulp
InactiveCN104082643AImprove protectionRefreshing tasteFood ingredient functionsFood preparationBiotechnologyGlucose lowering
The invention discloses a method for producing pumpkin pulp and belongs to the field of food processing. The method is characterized by adopting the processing process flows of selecting and cleaning materials, cutting and crushing, precooking and pulping, preparing and concentrating, canning and sterilizing, cooling and inspecting and finishing the product. The method has the beneficial effects that the product produced by the method is fresh and cool in taste and has a unique pumpkin flavor; the product is beneficial to gastric mucosa protection and helps digestion, has special treatment effects on the prevention and treatment of diabetes and the lowering of blood sugar and is convenient to eat and simple in operation.
Owner:谈茁
Chinese medicament for treating gastrosis and preparation method thereof
ActiveCN101940743AMeet modern requirementsImprove immunity against diseaseAnthropod material medical ingredientsDigestive systemCurative effectGastric mucosa
The invention relates to a Chinese medicament for treating gastrosis and a preparation method thereof. The Chinese medicament comprises the following medicaments in part by weight: 45 parts of rhizoma atractylodis macrocephalae, 45 parts of bletilla, 20 parts of astragals, 10 parts of hawthorn fruit, 10 parts of radish seed, 15 parts of chicken's gizzard-membrane, 15 parts of medicated leaven, 10 parts of malt, 20 parts of Indian buead, 30 parts of Mongolian dandelion herb, 20 parts of sea-buckthorn and 30 parts of propolis. The Chinese medicament of the invention has the main effect of protecting the gastric mucosa and restoring all functions of the gastric mucosa as soon as possible, and also has the effects of killing pathogenic bacteria, regulating the spleen and stomach, enhancing the anti-disease immune capability and the like; in addition, the Chinese medicament also has the advantages of high long-term taking safety, no toxic or side effect, high-quality materials, rational combination, scientific preparation, obvious curative effect, safety and reliability, stable quality and quick taking, and meets the modern requirements of Chinese medicinal preparations.
Owner:YANTAI MEISHILIN SHENGWU KEJI
Immunity-enhancing effervescent tablets and preparation method thereof
InactiveCN106552105AFix stability issuesSolve problems such as not easy to take for a long timeSenses disorderMetabolism disorderVitamin CIrritation
The invention discloses immunity-enhancing effervescent tablets. The effervescent tablets comprise the following components in parts by weight: 50-200 parts of vitamin C sodium, 10-30 parts of taurine, 10-30 parts of zinc gluconate and 10-20 parts of fig extract. In order to solve the problems that existing effervescent tablets comprising vitamin C are great in acidity, relatively great in irritation to oval cavity, throat and esophagus and gastric mucosa if being dissolved and not suitable for being taken for a long time, and vitamin C is likely to be oxidized to lose efficacy in the storage process, the effervescent tablets are likely to be affected with damp to absorb moisture and the like, the invention provides the effervescent tablets which have the functions of enhancing the immunity, clearing away heat and toxic materials, tonifying kidneys and spleen, maintaining beauty and keeping young and the like and further can supplement nutrients required by human body, relieve fatigue and delay senescence.
Owner:GUANGXI SANPOTEL HEALTH IND CO LTD
Sodium dichlorophenolate micro-pill pharmaceutical preparation and preparation method thereof
ActiveCN101804030ASustained releaseRelease stabilityOrganic active ingredientsAntipyreticMedicineCovering system
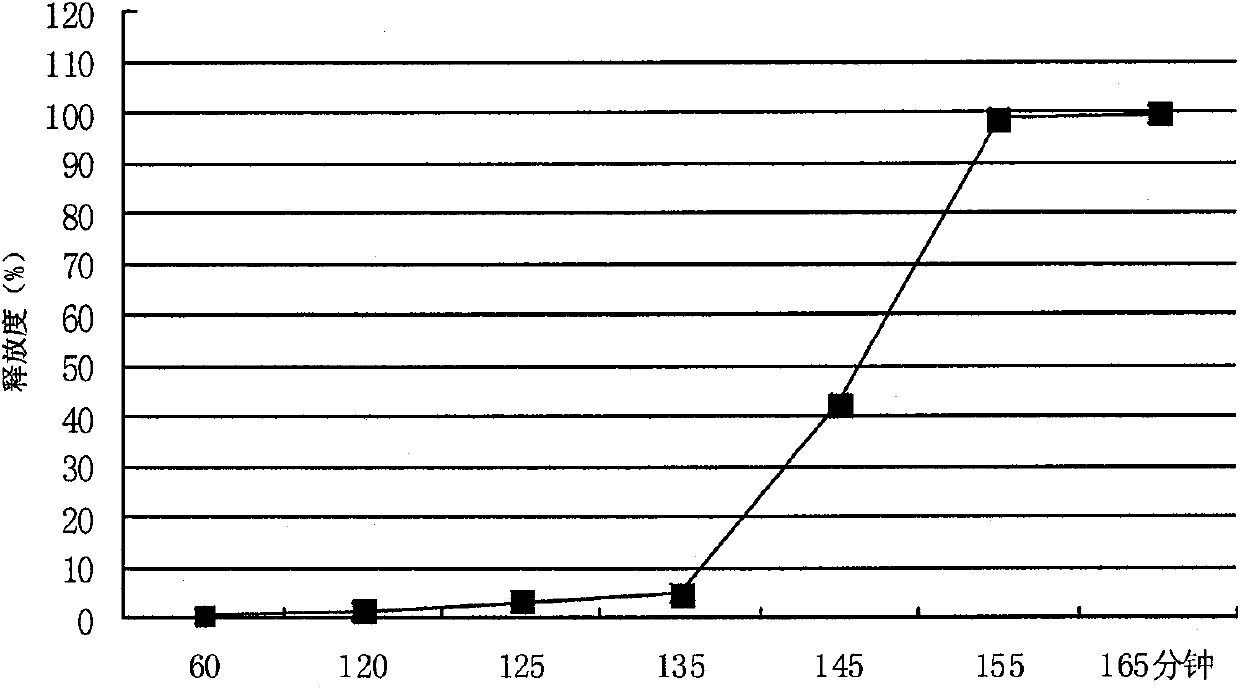
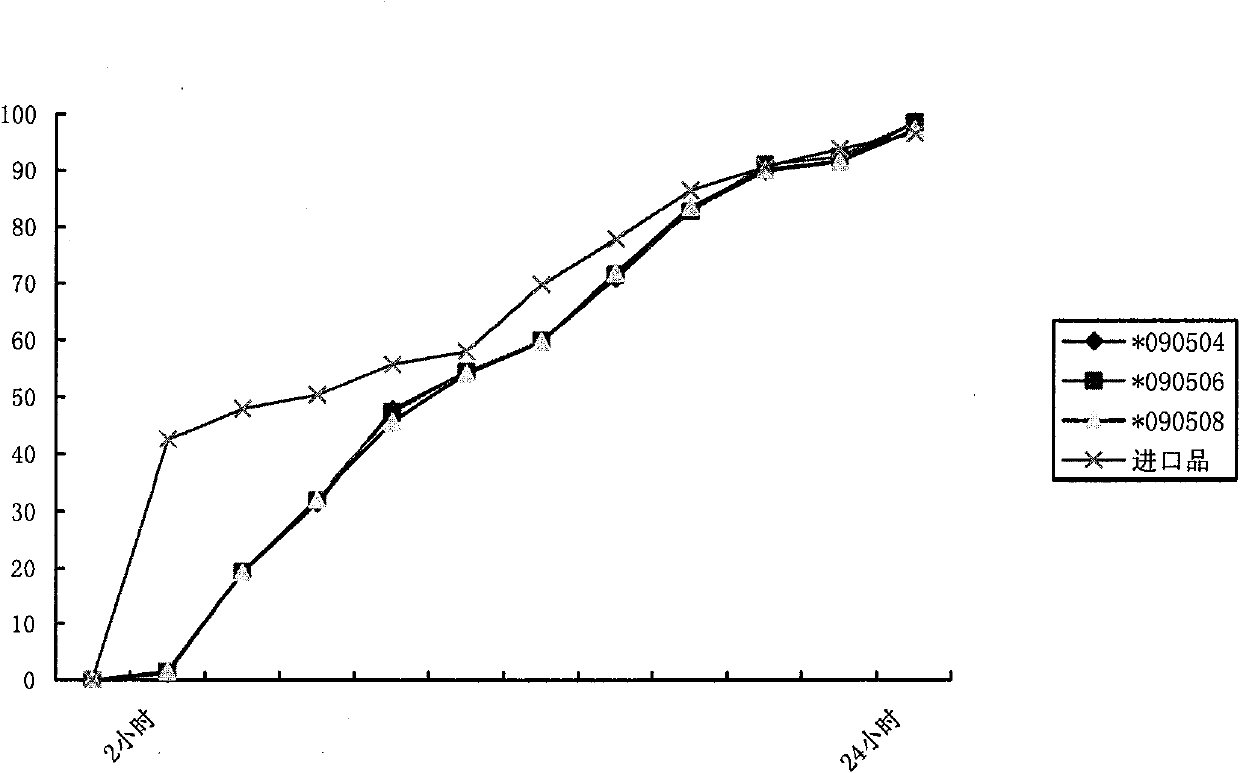
The invention relates to the pharmaceutical preparation field and in particular to a sodium dichlorophenolate micro-pill pharmaceutical preparation and a preparation method thereof. The micro-pill pharmaceutical preparation is prepared by micro-pills, which is characterized in that the micro-pill is of enteric slow-release micro-pill, the micro-pill consists of a hollow pill core, an active layer and an outer layer lagging cover, the active layer comprises sodium dichlorophenolate, a binding-property slow-release material and a binding-property enteric material, and the outer layer comprises a slow-release material. With the particular process and formula, a release system thereof is made into a unique double slow-release system, i.e. a skeleton-type scattering system is combined with a multi-layer semi-penetration film lagging cover system, so the main medicine can be continuously and stably released in the complicated internal environment of the human body. In addition, by adopting unique auxiliary materials, the micro-pill is free from being dissolved in the stomach and almost has no harm on the gastric mucosa, the medicine is ensured to have continuous effect of 24 hours inside the intestinal tract, the bioavailability is improved, and the application effect of the medicine is ensured.
Owner:HANGZHOU SHARPLY PHARM R&D INSTIT +1
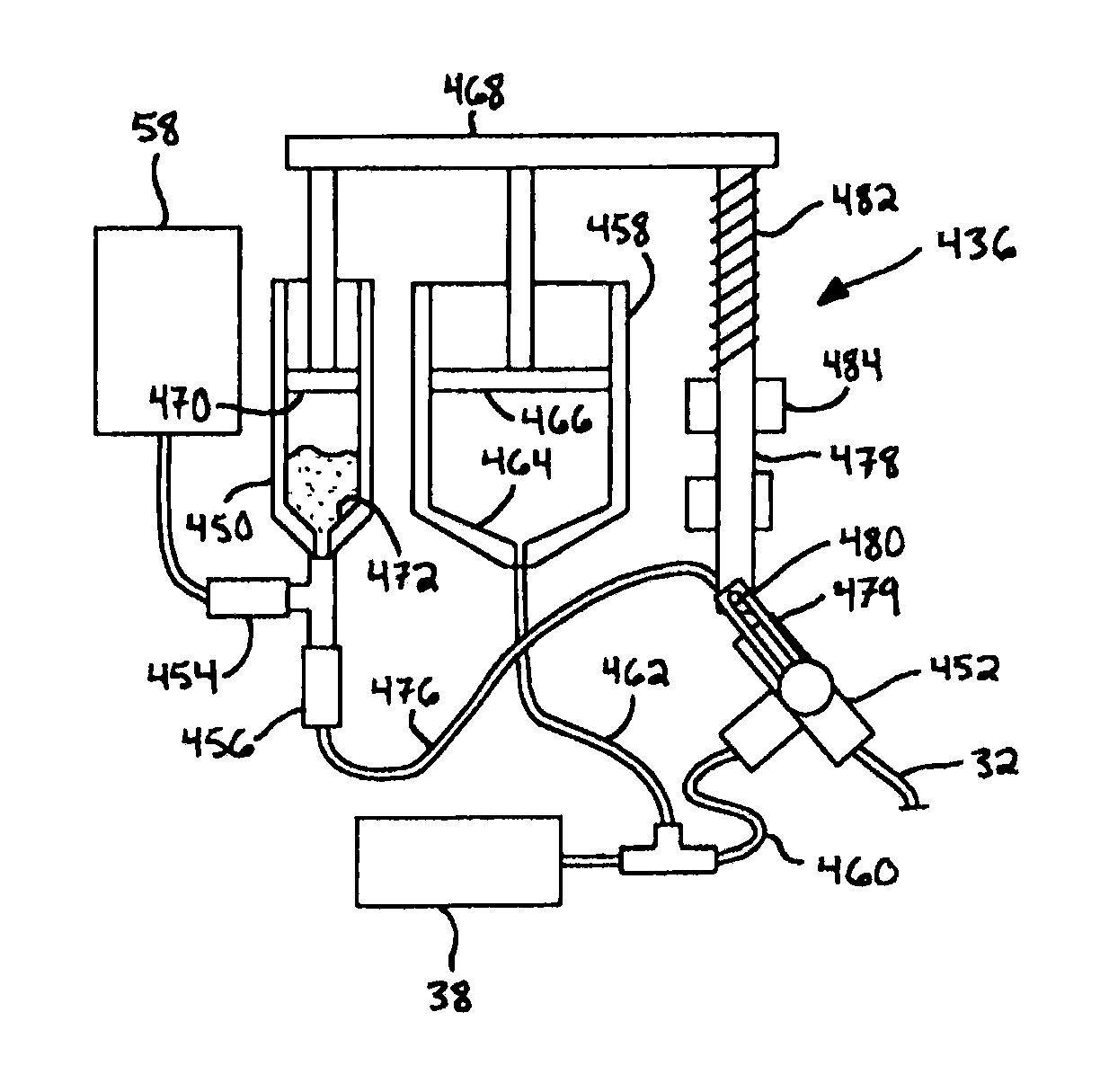
Medical aspiration apparatus
InactiveUS20140100531A1Quickly and easily alleviatingLower potentialInfusion devicesMedical devicesMedicineSolid particle
A medical aspiration apparatus for removing the contents of a subject's stomach includes a nasogastric (NG) tube having a tip structure that is less likely to be obstructed by stomach mucosa and solid particles of the stomach's contents. The apparatus also includes a flow control manifold that is capable of quickly and easily alleviating obstructions.
Owner:ANKRUM JAMES ALLEN +4
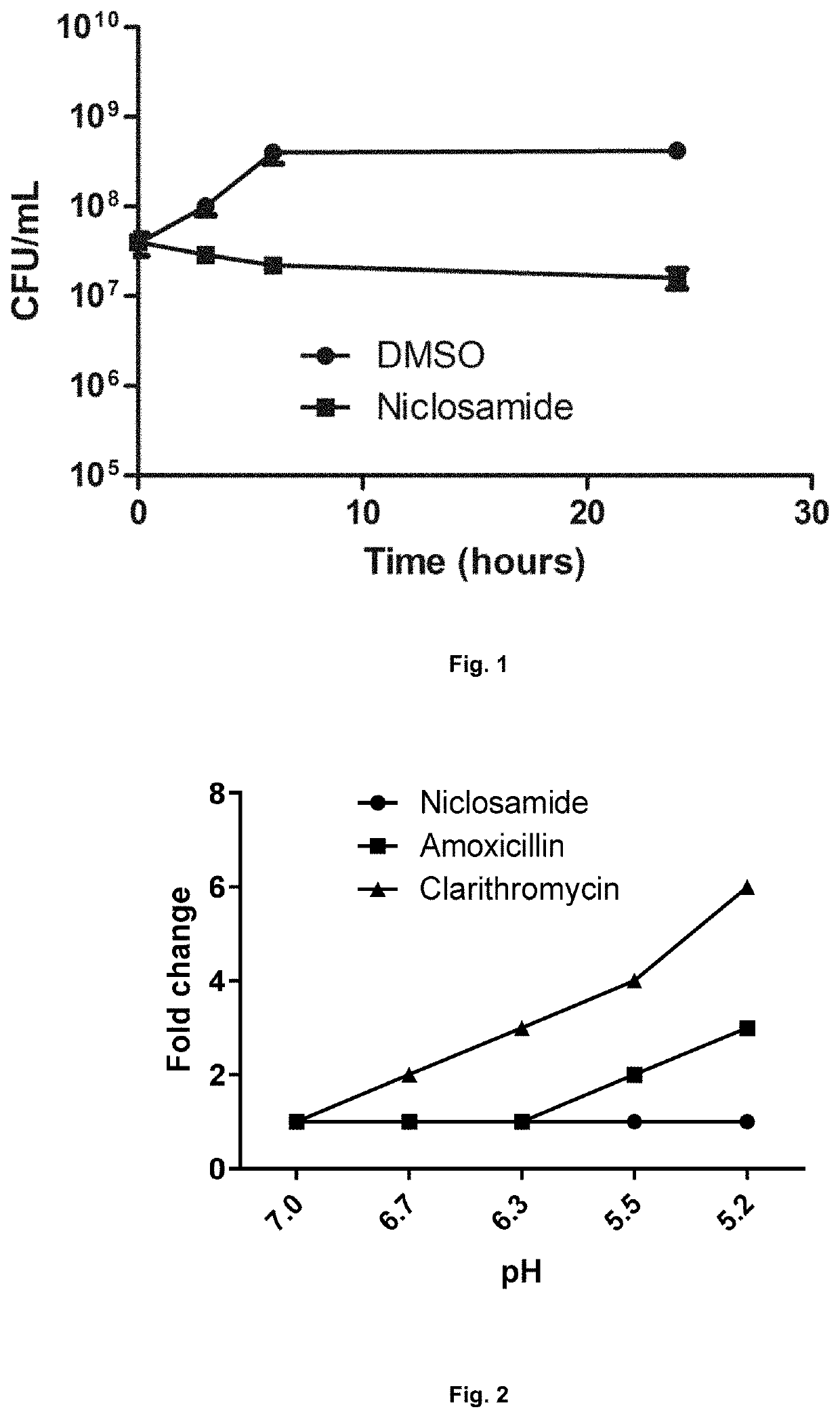
Methods for treating diseases or infections caused by or associated with h. pylori using a halogenated salicylanilide
ActiveUS20200268693A1Prevent and reduce riskInhibit and prevent progressionAntibacterial agentsOrganic active ingredientsDiseaseBiology
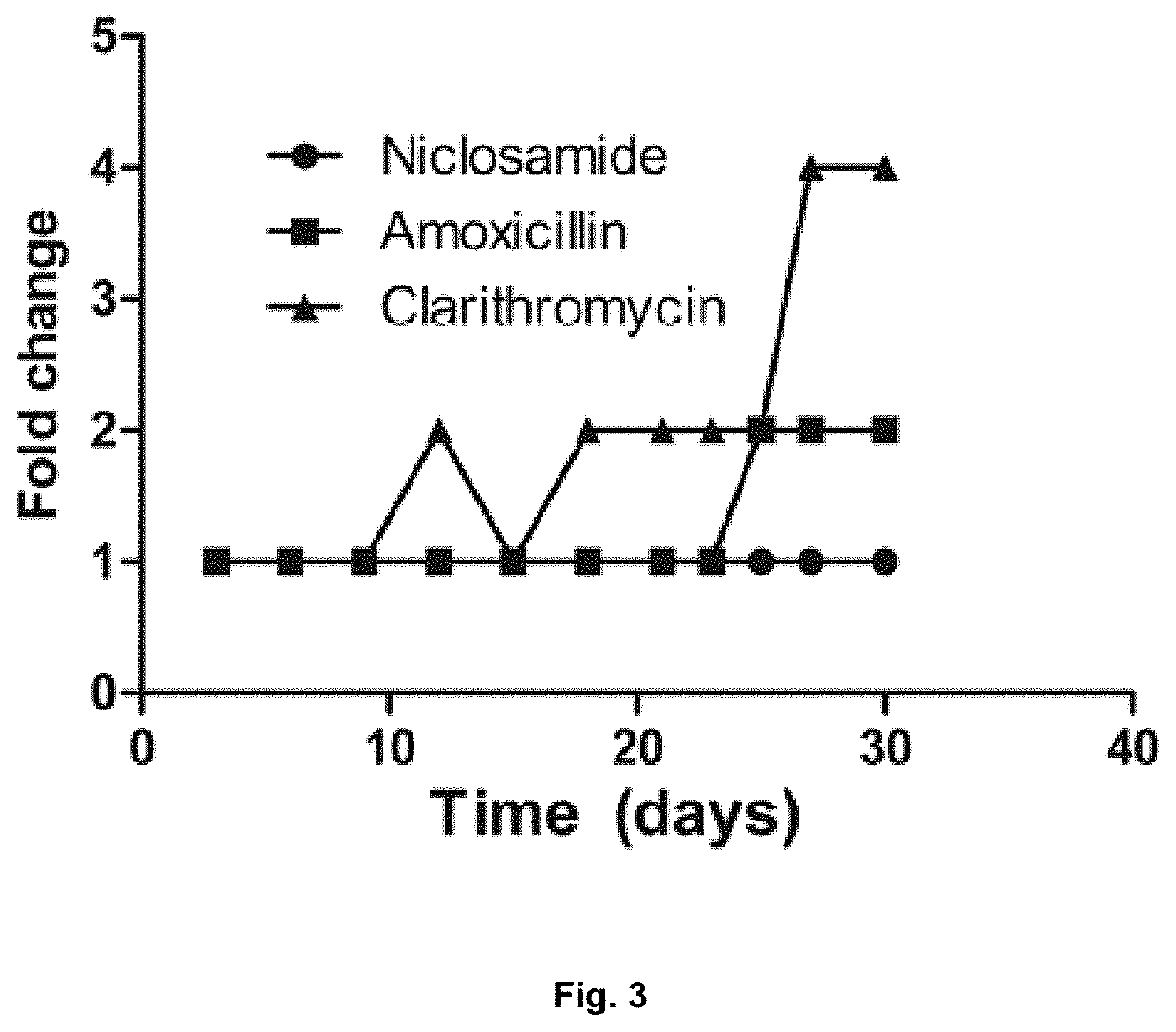
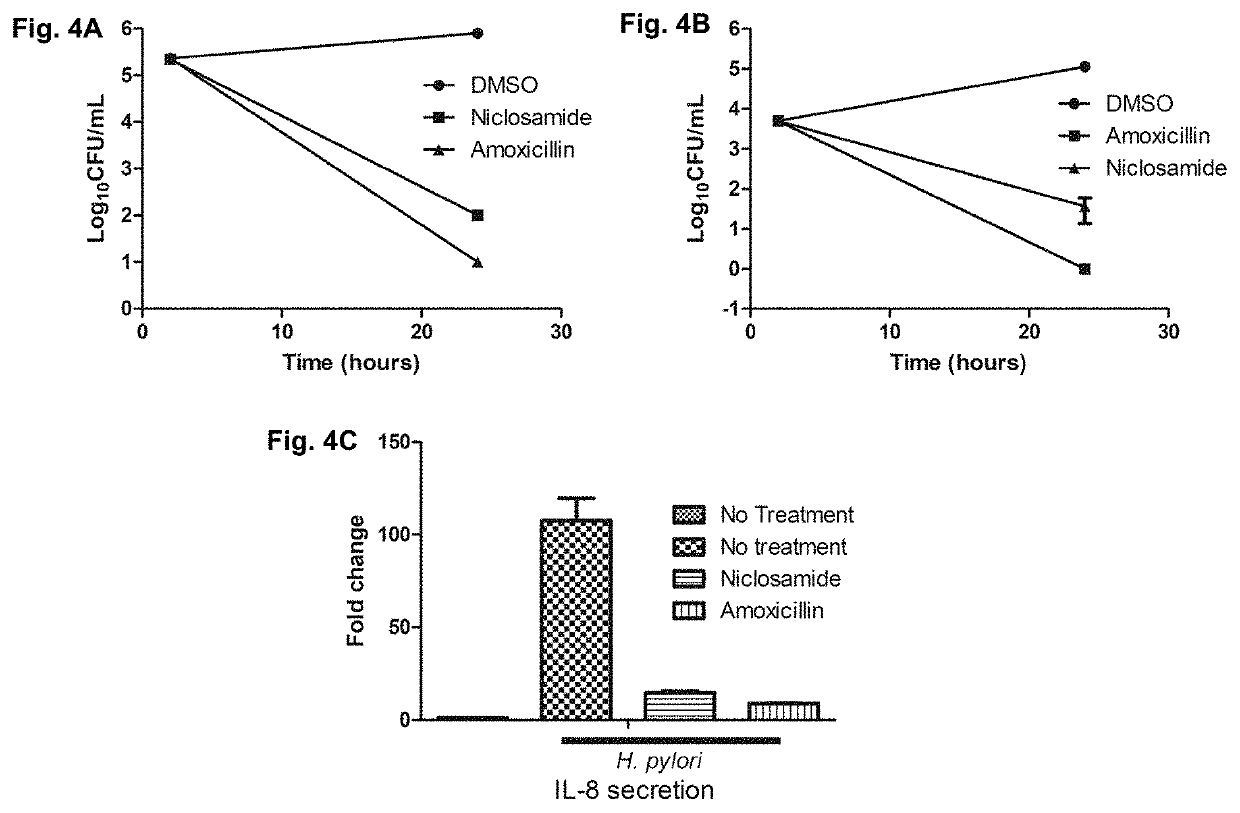
Disclosed are methods for the prevention or treatment of a disease or infection caused by or associated with H. Pylori in a subject infected by H. Pylori, the method comprising orally administering a halogenated salicylanilide such as niclosamide to the subject. The method may be used for the prevention or treatment of, for example dyspepsia, gastritis, peptic ulcer disease, premalignant gastric lesions, gastric cancer and gastric mucosa-associated lymphoid tissue (MALT) lymphoma.
Owner:RHODE ISLAND HOSPITAL
Oral liquid for treating chronic atrophic gastritis
InactiveCN103007206AReduce infiltrationInhibitory activityDigestive systemPharmaceutical delivery mechanismDiseaseArtemisia anomala
The invention belongs to the technical field of traditional Chinese medicines and relates to oral liquid for treating chronic atrophic gastritis. The oral liquid is prepared by the following components in parts by weight: 10 parts of Zhejiang white atractylodes rhizome, 15 parts of poria cocos, 10 parts of orange peel, 10 parts of pinellia tenata breit, 10 parts of vinegar rhizoma cyperi, 10 parts of fructus amomi, 10 parts of mangnolia officinalis, 10 parts of vinegar curcuma aromatic, 15 parts of artemisia anomala, 6 parts of cassia twig, 10 parts of rhizoma corydalis, 10 parts of hawthorn, 10 parts of roasted malt, 10 parts of roasted medicated leaven, 10 parts of lindera aggregate, 15 parts of radix paeoniae alba, 12 parts of radix pseudostellariae and 3 parts of liquorice. The oral liquid provided by the invention has the effects of relieving inflammatory cell infiltration, regulating cellular immunity and humoral immunity function, restraining the activity of pepsinogen to promote proliferation and recovery thereof, strengthening barrier protection function of the mucosa, regulating gastrointestinal motility, increasing permeability of gastric mucosa, improving blood circulation of the mucosa, promoting atrophic glands to recover, and delaying or reversing progression of diseases.
Owner:李郑生
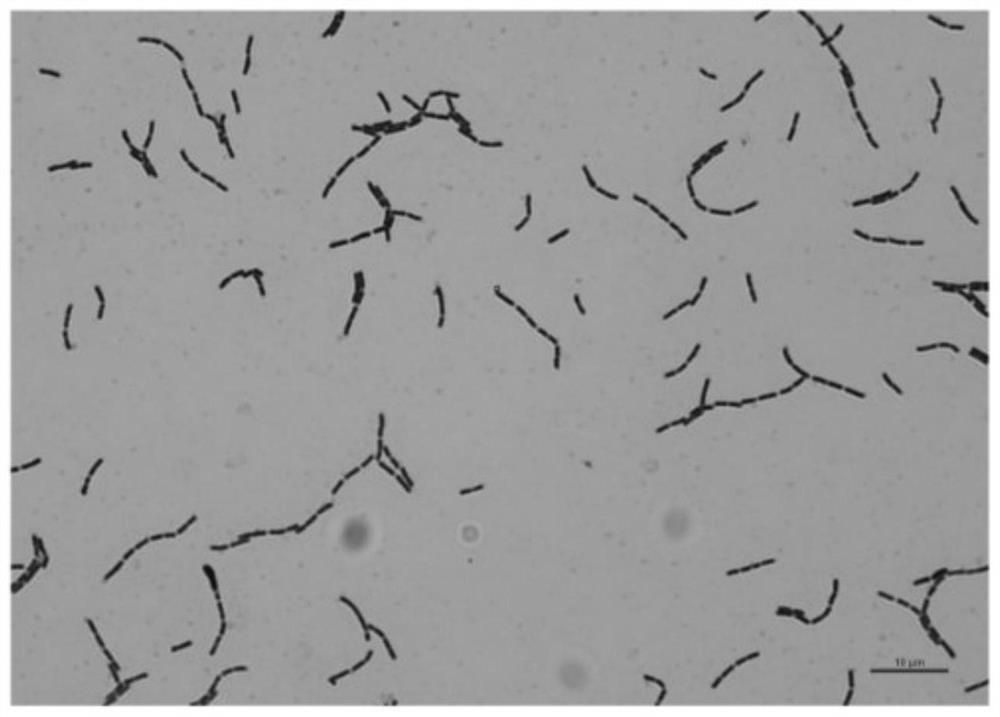
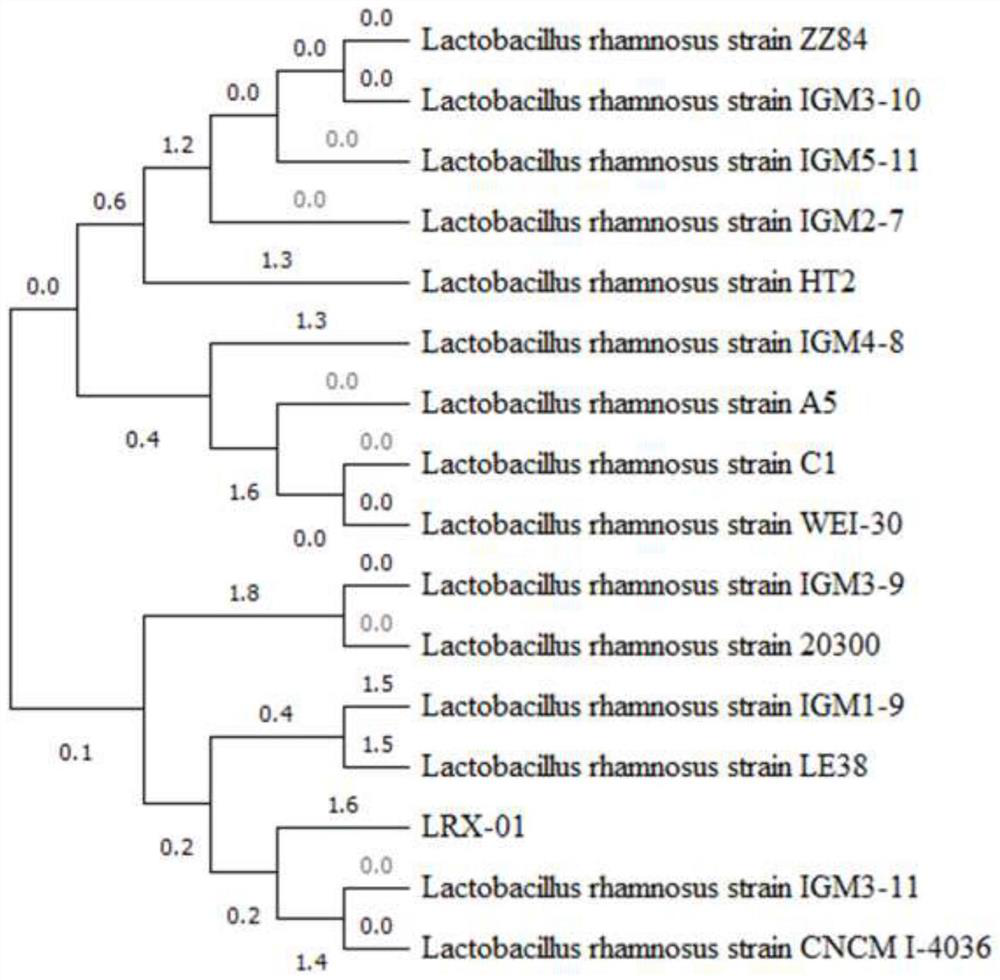
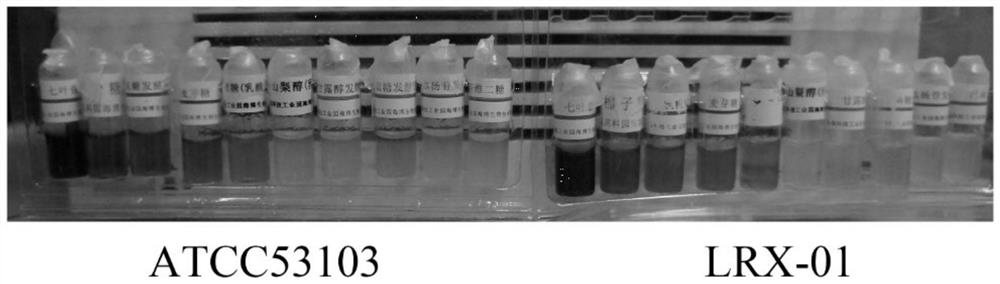
Lactobacillus rhamnosus and application thereof
ActiveCN113832077AImprove adhesionInhibition of Adhesive ColonizationAntibacterial agentsMilk preparationBiotechnologyLactobacillus rhamnosus
The invention discloses a strain of lactobacillus rhamnosus LRX-01, and the preservation number of the strain LRX-01 is GDMCC No: 60986. The lactobacillus rhamnosum LRX-01 disclosed by the invention has the general characteristics of probiotics, and has the advantages of good gastric acid resistance and good gastric mucosa adhesiveness. The lactobacillus rhamnosus LRX-01 can inhibit adhesion and colonization of helicobacter pylori in gastrointestinal tracts, can effectively inhibit growth of the helicobacter pylori, inhibits inflammatory response, helps to repair gastric mucosa tissues, and maintains microecological balance of the gastrointestinal tracts.
Owner:SOUTHERN MEDICAL UNIVERSITY +1
A pharmaceutical composition or health food composition for protecting gastric mucosa and its preparation method
The invention provides application of dendrobium aurantiacum Rchb. var. denneanum (Kerr)Z.H. Tsi or extract thereof to preparation of a medicine or health food for protecting gastric mucosa. The invention also provides a medicinal composition or a health food composition for protecting gastric mucosa. The medicinal composition or the health food composition is prepared from the following raw materials in part by weight: 3 to 7 parts of dendrobium aurantiacum Rchb. var. denneanum (Kerr)Z.H. Tsi, 1 to 3 parts of common bletilla pseudobulb, 3 to 7 parts of Chinese yam, 3 to 7 parts of tangerine peel, 3 to 7 parts of dandelion and 1 to 3 parts of liquorice. The invention also provides a preparation method and application of the medicinal composition. The medicine has the effect of protecting gastric mucosa, and a definite medicinal effect, and provides a new choice for clinic.
Owner:SICHUAN WANAN DENDROBIUM IND DEV
Traditional Chinese medicinal composition for protecting gastric mucosa and its preparation method
ActiveCN101120964AProtect gastric mucosaGood curative effectDigestive systemPill deliveryTreatment effectMedicine
The present invention discloses a Chinese medicine compound for gastric mucosa protection and the preparation method. The plantaginis and the dandelion are taken as the raw materials in the compound, which are mixed with the excipient into an oral preparation after extraction with the ethanol subsiding method or the alcohol extraction and decompression of the recycling recycling. The present invention has synergistic interaction function. Single application of the plantaginis and the dandelion both have gastric mucosa protection function, which have a synergistic interaction function after combination and the treatment effect can increase dramatically. The technique process is simple and convenient. The active part can be maintained as much as possible. The present invention has a good protection and treatment function for the acute gastric mucosa injury, the chronic gastric mucosa injury and the chronic atrophic gastritis.
Owner:ZHEJIANG YAKE SCI & TECH
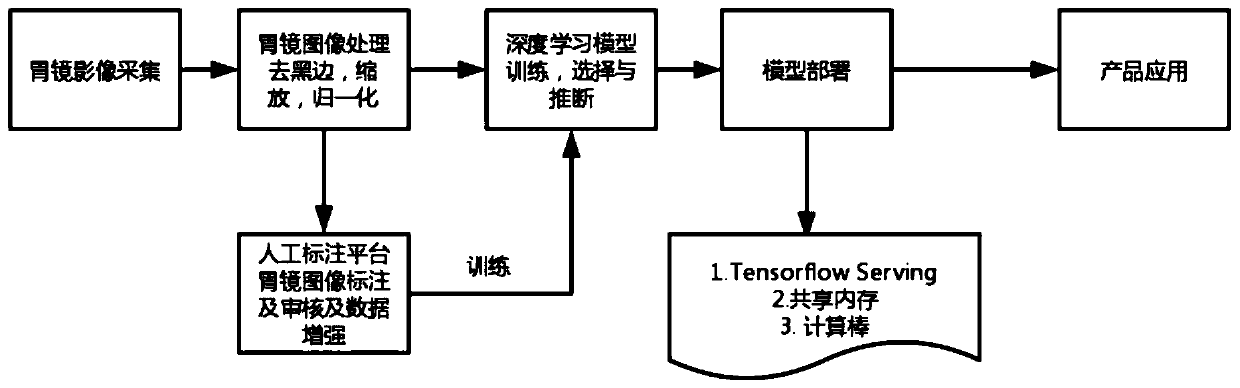
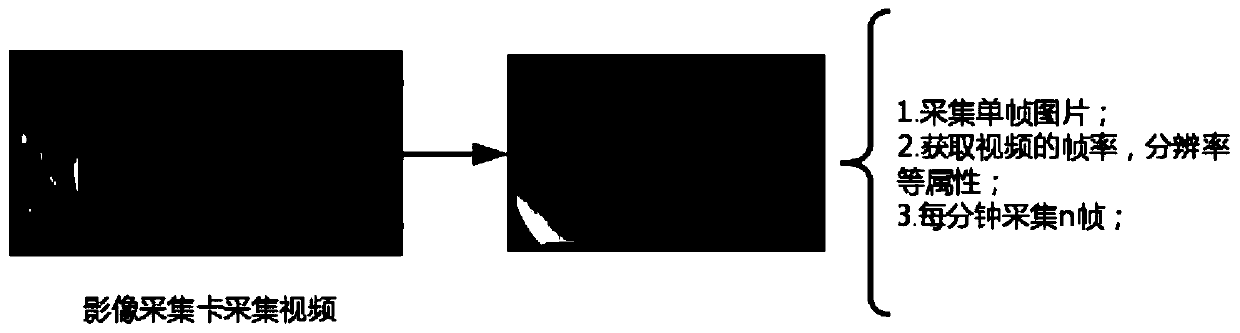
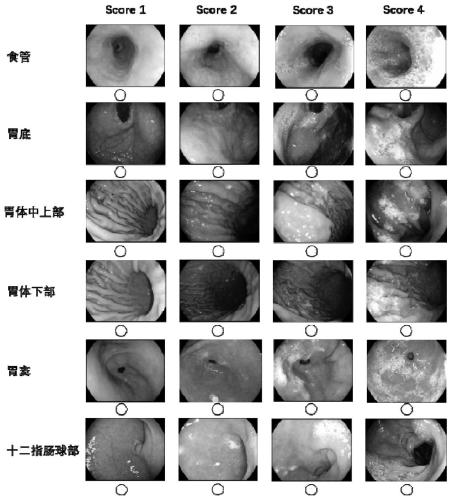
Gastric mucosa cleanliness evaluation method and system based on deep learning
ActiveCN111127426AAccurate assessment of cleanlinessImage enhancementImage analysisDiseaseComputer vision
The invention discloses a gastric mucosa cleanliness evaluation method and system based on deep learning. The method comprises the following steps: acquiring a gastroscope video frame in real time; performing cleanliness evaluation frame by frame based on a pre-constructed gastric mucosa cleanliness evaluation model; wherein a method for creating the gastric mucosa cleanliness evaluation model comprises the following steps of acquiring a gastroscope training image set; marking and scoring each training image according to a preset gastroscope cleanliness evaluation system; and training the gastric mucosa cleanliness evaluation model based on a deep learning network by adopting the training image set. The gastric mucosa cleanliness can be accurately evaluated, and a positive effect is achieved for diagnosis of gastric diseases and preparation and quality control before endoscopy.
Owner:SHANDONG UNIV QILU HOSPITAL +1
Preparation method of Chinese yam red bean cookies
InactiveCN104304382AImprove protectionSweet tasteDough treatmentBakery productsBiotechnologyDietary fiber
The invention discloses a preparation method of Chinese yam red bean cookies. The preparation method of the Chinese yam red bean cookies belongs to the field of food processing, and is characterized by adopting the processing technology process of sorting, peeling, washing and slicing, precooking, seasoning, sizing, drying, packaging and obtaining finished goods. The invention has the beneficial effects that the product is crispy, tasty, fragrant and sweet, and has the specific flavors of Chinese yam and red bean. The product is rich in dietary fiber, is beneficial to protection of gastric mucosa, is helpful to digestion, has the efficacies of nourishing yin and lung and reducing blood pressure and blood glucose, and is convenient to eat, and simple to operate.
Owner:杨燕
Enzyme beverage with healthcare effects of strengthening spleen and nourishing stomach and preparation process thereof
InactiveCN107373255AHas the effect of invigorating the spleen and nourishing the stomachMild action and effectNatural extract food ingredientsFood ingredient functionsMicroorganismReflux esophagitis
The invention provides an enzyme beverage with healthcare effects of strengthening the spleen and nourishing the stomach and a preparation process thereof. A unique traditional Chinese medicine secret recipe is taken as a raw material for fermenting to prepare a live-bacteria enzyme beverage. The enzyme beverage has the effects of repairing gastric mucosa fall-off and treating reflux esophagitis, chronic gastritis and gastric and duodenal ulcer, and is beneficial for people suffering from stomach trouble. The reasonable traditional Chinese medicine formula is combined with the enzyme technology, active ingredients of Chinese herbal medicines are enriched and purified through action of microorganisms, and co-act with regulatory factors such as enzyme to achieve better effect, and therefore, the enzyme beverage is safe, healthy, mild in acting effect, and has the healthcare effects of strengthening the spleen and nourishing the stomach.
Owner:SHANDONG BOHUA HIGHLY EFFICIENT ECOLOGICAL & AGRI TECH CO LTD
Preparation process for apricot and Chinese yam biscuits
InactiveCN104996521AImprove protectionReduce heatDough treatmentBakery productsBiotechnologyDigestion
The invention discloses a preparation process for apricot and Chinese yam biscuits and belongs to the field of food processing. The preparation process is characterized by comprising the following process flows: preparing paste; rolling the paste to form; roasting the paste; cooling the paste; and packaging the paste by taking 16kg of Chinese yam whole flour, 7kg of corn starch, 25kg of high gluten flour, 5kg of wheat meal, 3kg of black sesame oil, 4kg of eggs, 20kg of brown sugar, 850g of apricots and a proper amount of rose essence, sodium hydrogen carbonate and ammonium hydrogen carbonate. The preparation process has the benefits that the product is slightly yellow in color and luster, crispy and tasty and strong in flavor, and has a flavor of Chinese yam which is fragrant and lubricant. The product is rich in dietary fiber, the gastric mucosa is protected, digestion is helped, and the product further has the functions of nourishing yin and lung and reducing blood pressure and blood glucose and is a rare health-care food which is low in heat, low in sugar and low in fat.
Owner:江荧
Technology for processing health care rhizoma dioscoreae soft sweets
The invention discloses technology for processing health care rhizoma dioscoreae soft sweets, belonging to the field of food processing. The technology is characterized by adopting rhizoma dioscoreae powder, barbary wolfberry fruit powder, carrageenan, maltose, stevioside and strawberry essence as raw materials in a formula. The technology has the beneficial effects that the products taste fragrant, sweet and strong, have delicate and soft taste, have agreeable sweetness and have the fragrant and sweet flavor of rhizoma dioscoreae; the products are rich in dietary fibers, are beneficial to protecting the gastric mucosa and helping digestion, also have the functions of nourishing yin and the lung and reducing blood pressure and blood sugar, and are rare low-calorie, low-sugar and low-fat health food.
Owner:谈茁
Composition for relieving stomach illness and preparation method thereof and food for relieving stomach illness
PendingCN111011856AEnhanced inhibitory effectPromote recoveryDigestive systemUnknown materialsHelicobacterBroccoli raab
The invention relates to the technical field of functional foods, in particular to a composition for relieving stomach illness and a preparation method thereof and a food for relieving stomach illness. The composition is prepared from the following main materials in parts by weight: 2.5 to 7.5 parts of probiotics, 2 to 3 parts of broccoli seed water extract, 10 to 25 parts of fermented soybean meal and 5 to 10 parts of Korean vegetable water extract concentrate. The composition has a good inhibition effect on helicobacter pylori, can relieve hyperacidity and repair ulcer, and is beneficial torecovery of gastric mucosa.
Owner:NANJING SHENGNUO BIO TECH IND
Chinese yam and poria cocos bread production technology
InactiveCN105053114AImprove protectionYellowish colorDough treatmentBakery productsBiotechnologyPolygonum fagopyrum
The present invention discloses a Chinese yam and poria cocos bread production technology, which belongs to the field of food processing. The Chinese yam and poria cocos bread is characterized by comprising the following raw materials low-gluten flour 35 kg, buckwheat flour 10 kg, Chinese yam powder 15 kg, poria cocos powder 8 kg, yeast 0.6 kg, sucrose 12 kg, edible salt 0.5 kg, and soybean oil 0.9 kg. The production processes are as follows the flour, Chinese yam and poria cocos are pretreated, the bread is made through the procedures of material stirring, fermentation, shaping, proofing and baking, and the obtained bread is taken out of the oven, cooled and packaged. The beneficial effects are as follows the product is yellowish in color and luster, and mellow in fragrance, soft, fragrant and crisp in mouthfeel, and has light, fragrant and glutinous flavors of the Chinese yam and poria cocos. The Chinese yam and poria cocos bread is rich in dietary fibers, is conductive for gastric mucosa protection and digestion, also has efficacies of nourishing yin and tonifying lungs, and lowering blood pressure and blood sugar, and is a rare health-care food which is low-calorie, low-sugar, and low-fat.
Owner:卢国孝
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com