Voglibose tablet and preparation method thereof
A technology of voglibose and sugar tablets, which is applied in the field of oral preparations containing voglibose and its preparation, can solve the problem of difficulty in realizing large-scale industrial production, reducing the absorption rate of voglibose, and affecting the production of voglibose. Glibose curative effect and other issues, to achieve the effect of fewer steps, improved bioavailability, and rapid dissolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
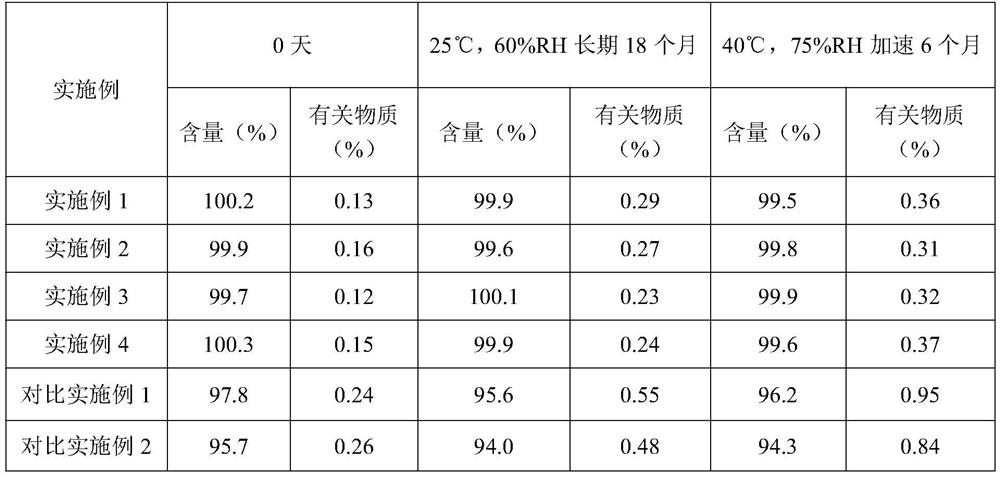
Embodiment 1
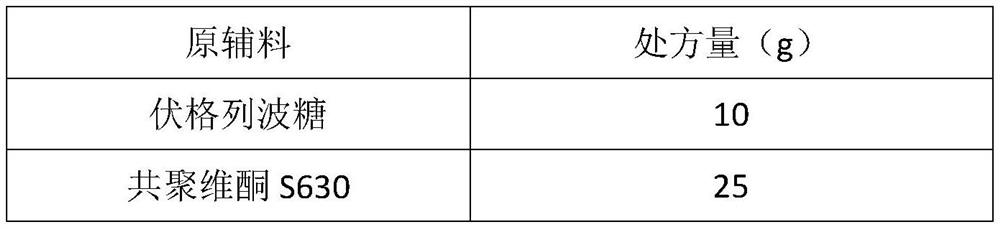
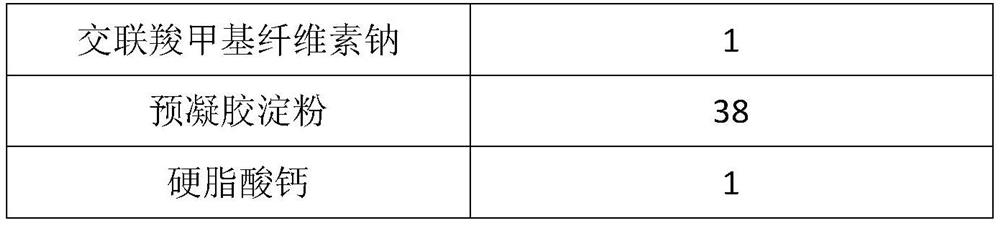
[0027] Raw materials Prescription amount (g) Voglibose 10 Copovidone VA64 25 Croscarmellose Sodium 2 porous starch 40 Sodium stearyl fumarate 1
[0028] Preparation:
[0029] Weigh the raw materials and copovidone VA64 according to the prescription amount, heat and melt the extrusion at 151°C through a hot-melt extruder, crush the extrudate, pass through a 80-mesh sieve, and mix with the prescription amount of croscarmellose sodium , porous starch, and sodium stearyl fumarate are mixed evenly, and the tablet hardness is controlled to 30N, and the tablets are directly pressed.
Embodiment 2
[0031] Raw materials Prescription amount (g) Voglibose 5 Copovidone S630 10 Carmellose Calcium 1 porous starch 25 Magnesium stearate 0.5
[0032] Preparation:
[0033] Weigh the raw materials and copovidone S630 according to the prescription amount, heat and melt the extruded product at 151°C through a hot-melt extruder, crush the extrudate, pass through a 80-mesh sieve, and mix with carmellose calcium, porous starch, stearin The magnesium acid is mixed evenly, and the tablet hardness is controlled to be 30N, and the tablets are directly compressed.
Embodiment 3
[0035] Raw materials Prescription amount (g) Voglibose 5 Copovidone VA64 15 Hydroxypropyl starch 1 porous starch 34 talcum powder 0.5
[0036] Preparation:
[0037] Weigh the raw materials and copovidone VA64 according to the prescription amount, heat and melt the extruded product at 151°C through a hot-melt extruder, crush the extrudate, pass through a 80-mesh sieve, and mix with hydroxypropyl starch, porous starch, and talc powder Uniform, controlled tablet hardness 30N, direct tablet compression.
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com