Preparation method of voglibose capsule
A technology for voglibose and capsules, which is applied in the field of pharmaceutical preparations, can solve the problems of poor voglibose content uniformity, low dissolution rate, poor voglibose content uniformity and the like, and achieves inhibition of crystallization. Phenomenon, good uniformity, good dissolution and effect of content uniformity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
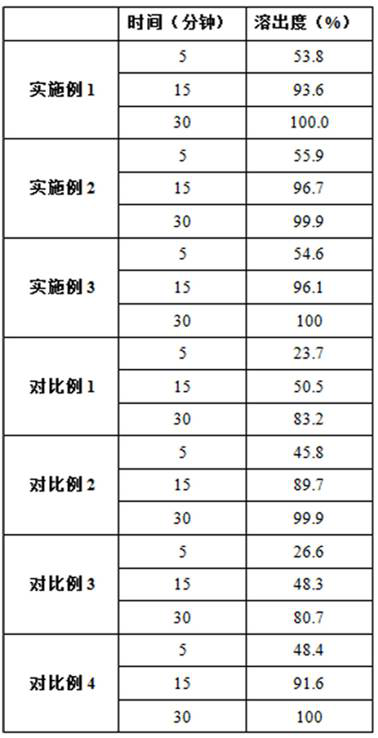
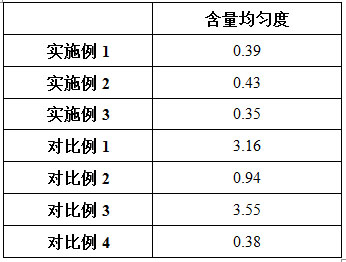
Embodiment 1
[0032] Embodiment 1: a kind of preparation method of voglibose capsules
[0033] Include the following steps:
[0034] 1. Preparation of the original drug solution
[0035] Add voglibose to deionized water, after the dissolution is complete, add 20 polyglycerol monostearate, polyvinyl pyrrolidone, polyethylene glycol 15-hydroxystearate, oat beta glucan in turn, stir After the dissolution is complete, the micropowder silica gel is slowly added, and after the addition is completed, it is fully stirred to be in a state of suspension to obtain the original drug solution;
[0036] The molecular weight of the polyvinylpyrrolidone is 8800g / mol;
[0037] The molecular weight of the polyethylene glycol 15-hydroxystearate is 963.2 g / mol;
[0038] The molecular weight of the oat beta glucan is 10530g / mol;
[0039] The particle size of the micropowder silica gel is 5 microns, and the specific surface area is 270 m 2 / g;
[0040] The mass ratio of described voglibose, deionized water...
Embodiment 2
[0049] Embodiment 2: a kind of preparation method of voglibose capsules
[0050] Include the following steps:
[0051] 1. Preparation of the original drug solution
[0052] Add voglibose to deionized water, after the dissolution is complete, add 20 polyglycerol monostearate, polyvinyl pyrrolidone, polyethylene glycol 15-hydroxystearate, oat beta glucan in turn, stir After the dissolution is complete, the micropowder silica gel is slowly added, and after the addition is completed, it is fully stirred to be in a state of suspension to obtain the original drug solution;
[0053] The molecular weight of the polyvinylpyrrolidone is 6000g / mol;
[0054] The molecular weight of the polyethylene glycol 15-hydroxystearate is 963.2 g / mol;
[0055] The molecular weight of the oat beta glucan is 6300 g / mol;
[0056] The particle size of the micropowder silica gel is 3 microns, and the specific surface area is 150 m 2 / g;
[0057] The mass ratio of described voglibose, deionized water...
Embodiment 3
[0066] Embodiment 3: a kind of preparation method of voglibose capsules
[0067] Include the following steps:
[0068] 1. Preparation of the original drug solution
[0069] Add voglibose to deionized water, after the dissolution is complete, add 20 polyglycerol monostearate, polyvinyl pyrrolidone, polyethylene glycol 15-hydroxystearate, oat beta glucan in turn, stir After the dissolution is complete, the micropowder silica gel is slowly added, and after the addition is completed, it is fully stirred to be in a state of suspension to obtain the original drug solution;
[0070] The molecular weight of the polyvinylpyrrolidone is 10000g / mol;
[0071] The molecular weight of the polyethylene glycol 15-hydroxystearate is 963.2 g / mol;
[0072] The molecular weight of the oat beta glucan is 12000 g / mol;
[0073] The particle size of the micropowder silica gel is 8 microns, and the specific surface area is 360 m 2 / g;
[0074] The mass ratio of described voglibose, deionized wat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com