Metformin hydrochloride enteric-coated tablet
A metformin hydrochloride enteric and tablet-dissolving technology, which is applied to medical preparations of non-active ingredients, metabolic diseases, organic active ingredients, etc., can solve the problems of unguaranteed curative effect and complicated preparation process, and achieve saving of enteric coating materials and preparation The effect of process simplification, raw material cost and production cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
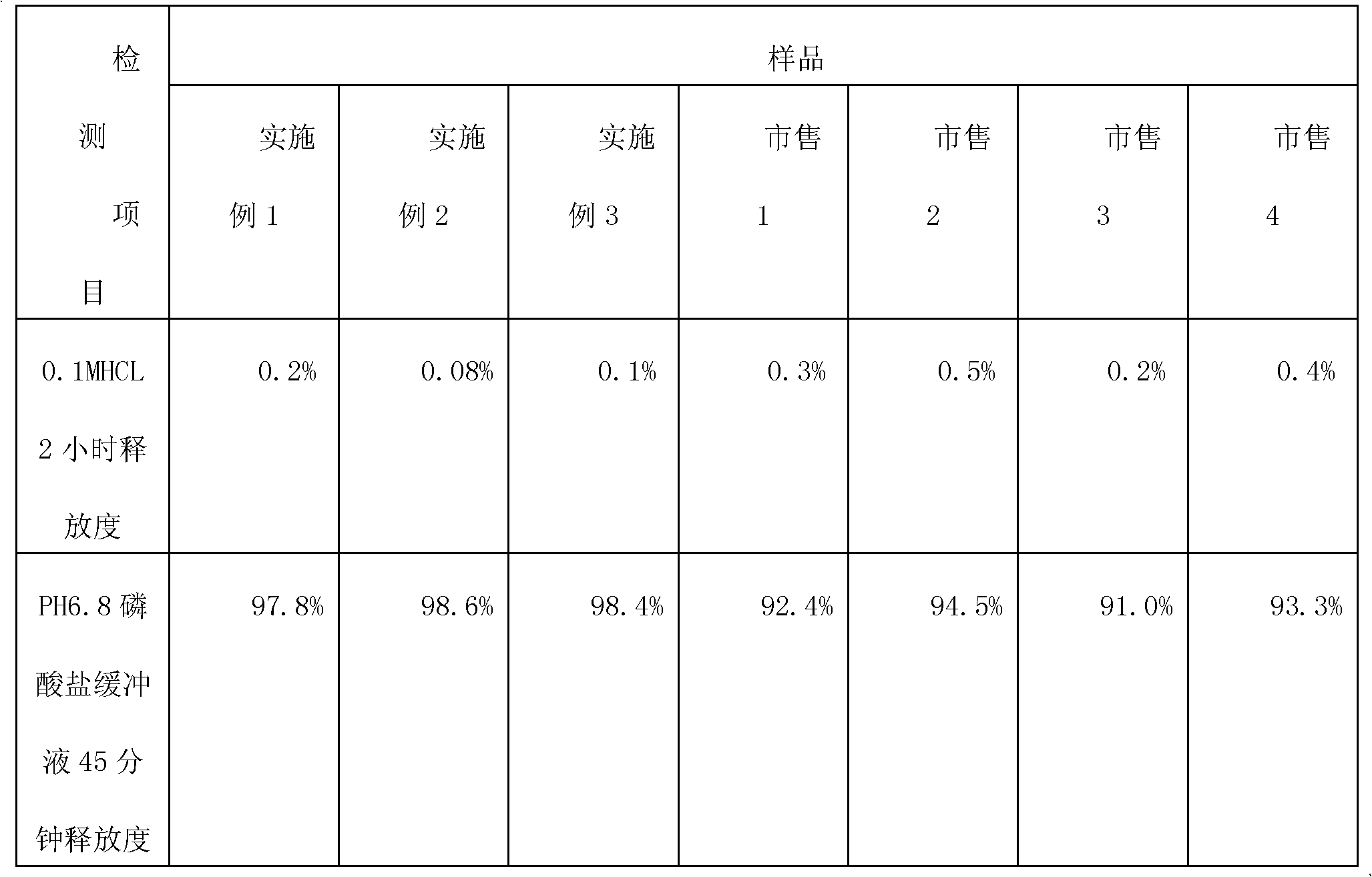
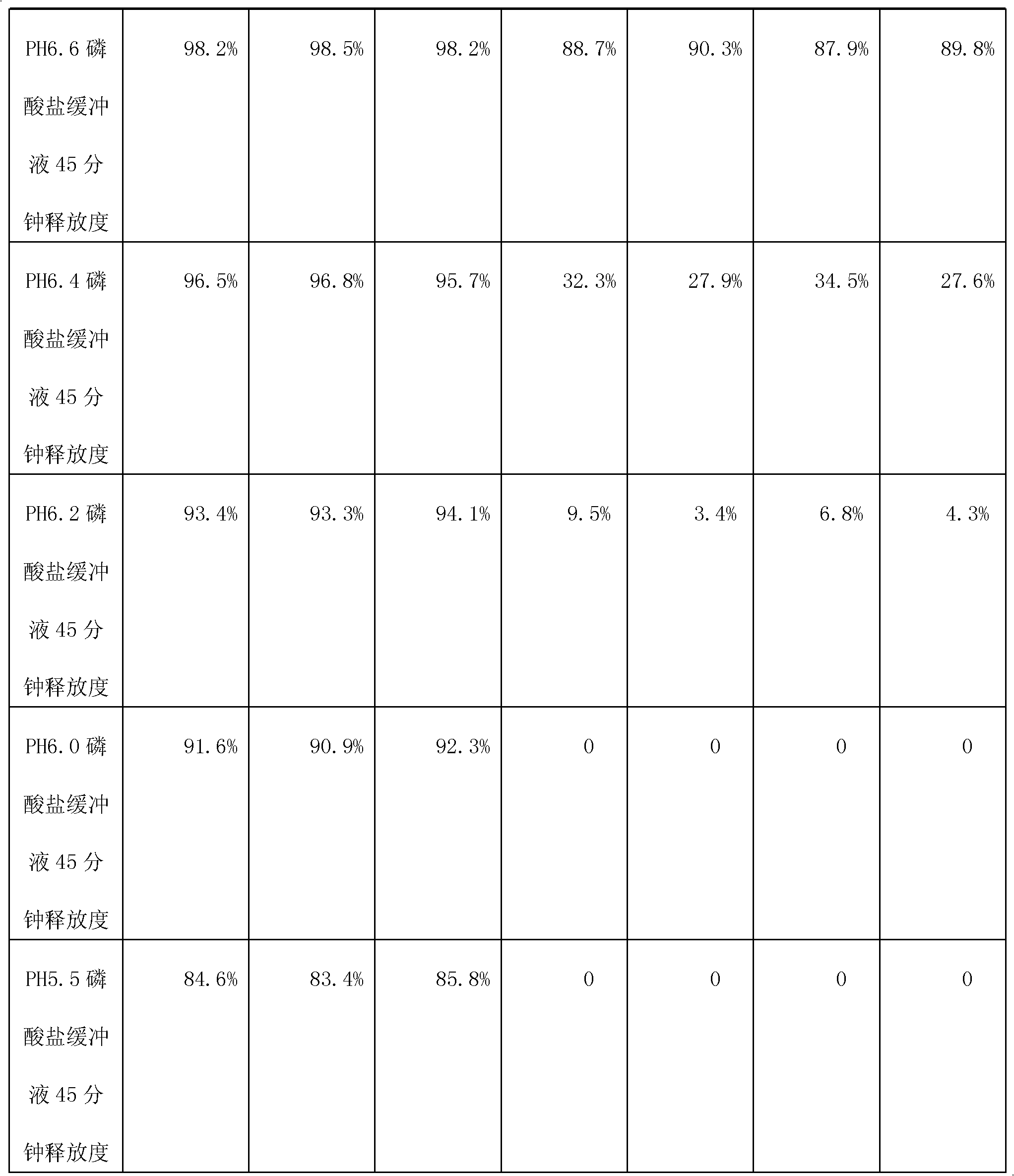
[0026]The specification is 0.25g, based on 10,000 tablets, the tablet core is made of 2500g of metformin hydrochloride, 3075g of povidone K30, 300g of 75% ethanol aqueous solution containing 10% of povidone K30, and 25g of magnesium stearate; The coating layer is made of Eudragit L30D-55250g, 37.5g of talcum powder, 7.5g of PEG4000, 12.5g of 4% sodium hydroxide solution, and 250g of purified water. 150g of enteric coating liquid is used for coating every 1kg of tablet core.
[0027] Described metformin hydrochloride enteric-coated tablet is made according to the following steps:
[0028] (1) weighing each raw material in proportion;
[0029] (2) Tablet core preparation: after metformin hydrochloride is pulverized, put in the high-efficiency wet mixing granulator, add the internally added povidone K30 of recipe quantity, after stirring and mixing evenly, add containing povidone K30 to be 10% (wt) %) of 75% (wt%) ethanol aqueous solution is prepared into a suitable soft materi...
Embodiment 2
[0033] Based on 5000 tablets, the specification is 0.5g, and the tablet core is made of 2500g of metformin hydrochloride, 125g of povidone K30, 200g of 75% ethanol aqueous solution with binder 10% of povidone K30, and 12.5g of magnesium stearate; The enteric coating layer is made of Eudragit L30D-55250g, talcum powder 25g, PEG40005g, 4% sodium hydroxide solution 15g and purified water 300g. 170g of enteric coating liquid is used for coating every 1kg of tablet core.
[0034] Preparation:
[0035] (1) weighing each raw material in proportion;
[0036] (2) Tablet core preparation: after metformin hydrochloride is pulverized, put in the high-efficiency wet mixing granulator, add the internally added povidone K30 of recipe quantity, after stirring and mixing evenly, add containing povidone K30 to be 10% (wt) %) of 75% (wt%) ethanol aqueous solution is prepared into a suitable soft material, prepared into granules through a nylon sieve, mixed evenly with magnesium stearate after ...
Embodiment 3
[0040] In terms of making 10000 tablets, the specification is 0.85g, and the tablet core is made of 8500g of metformin hydrochloride, 340g of povidone K30, 850g of 75% ethanol aqueous solution with binder 10% of povidone K30, and 68g of magnesium stearate; The enteric coating layer is made of Eudragit L30D-55250g, talcum powder 75g, PEG400010g, 4% sodium hydroxide solution 10g, purified water 200g. 158g of enteric coating solution is used for coating every 1kg tablet core.
[0041] Preparation:
[0042] (1) weighing each raw material in proportion;
[0043] (2) Tablet core preparation: after metformin hydrochloride is pulverized, put in the high-efficiency wet mixing granulator, add the internally added povidone K30 of recipe quantity, after stirring and mixing evenly, add containing povidone K30 to be 10% (wt) %) of 75% (wt%) ethanol aqueous solution is prepared into a suitable soft material, prepared into granules through a nylon sieve, mixed evenly with magnesium stearate...
PUM
| Property | Measurement | Unit |
|---|---|---|
| release amount | aaaaa | aaaaa |
| release amount | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com