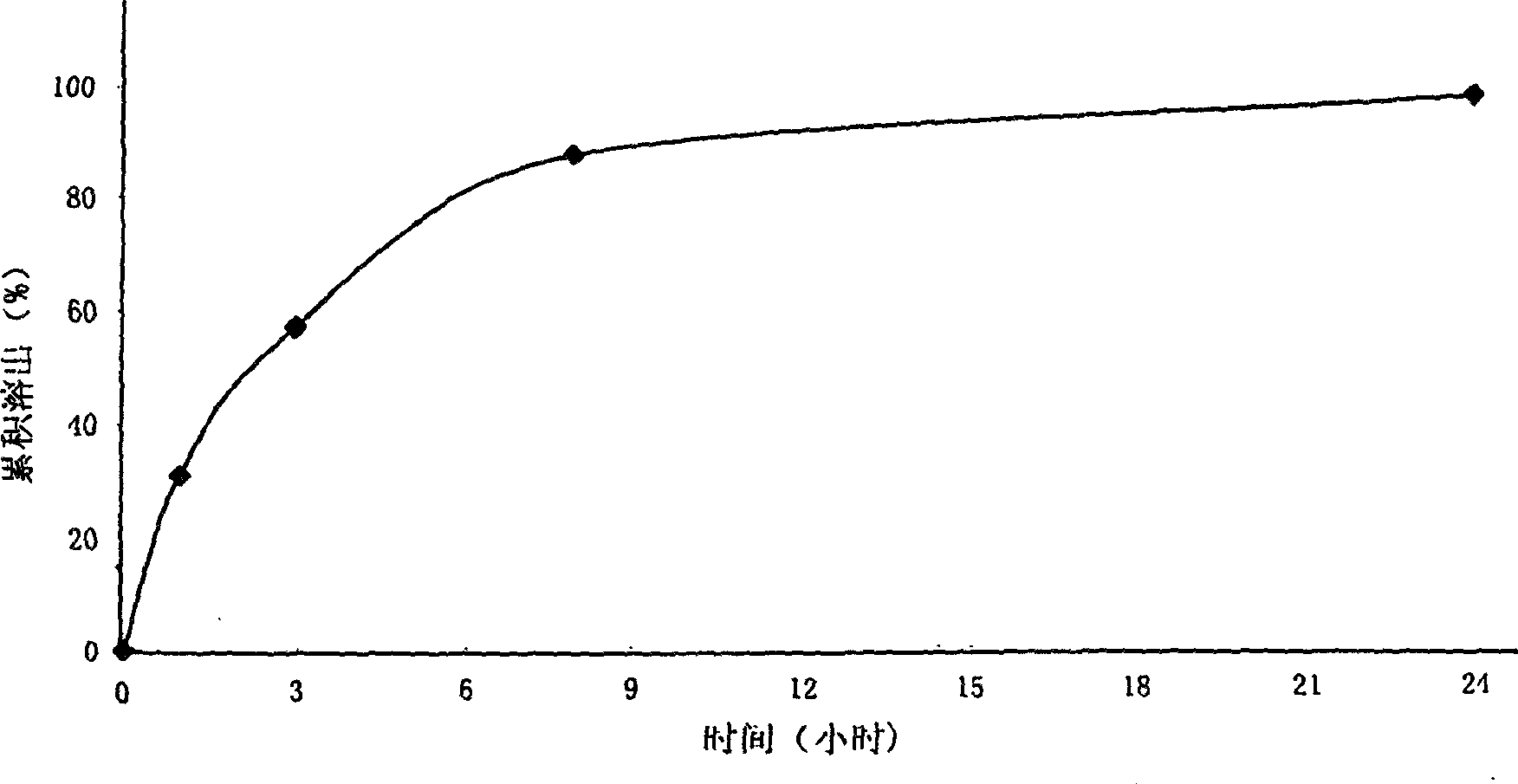
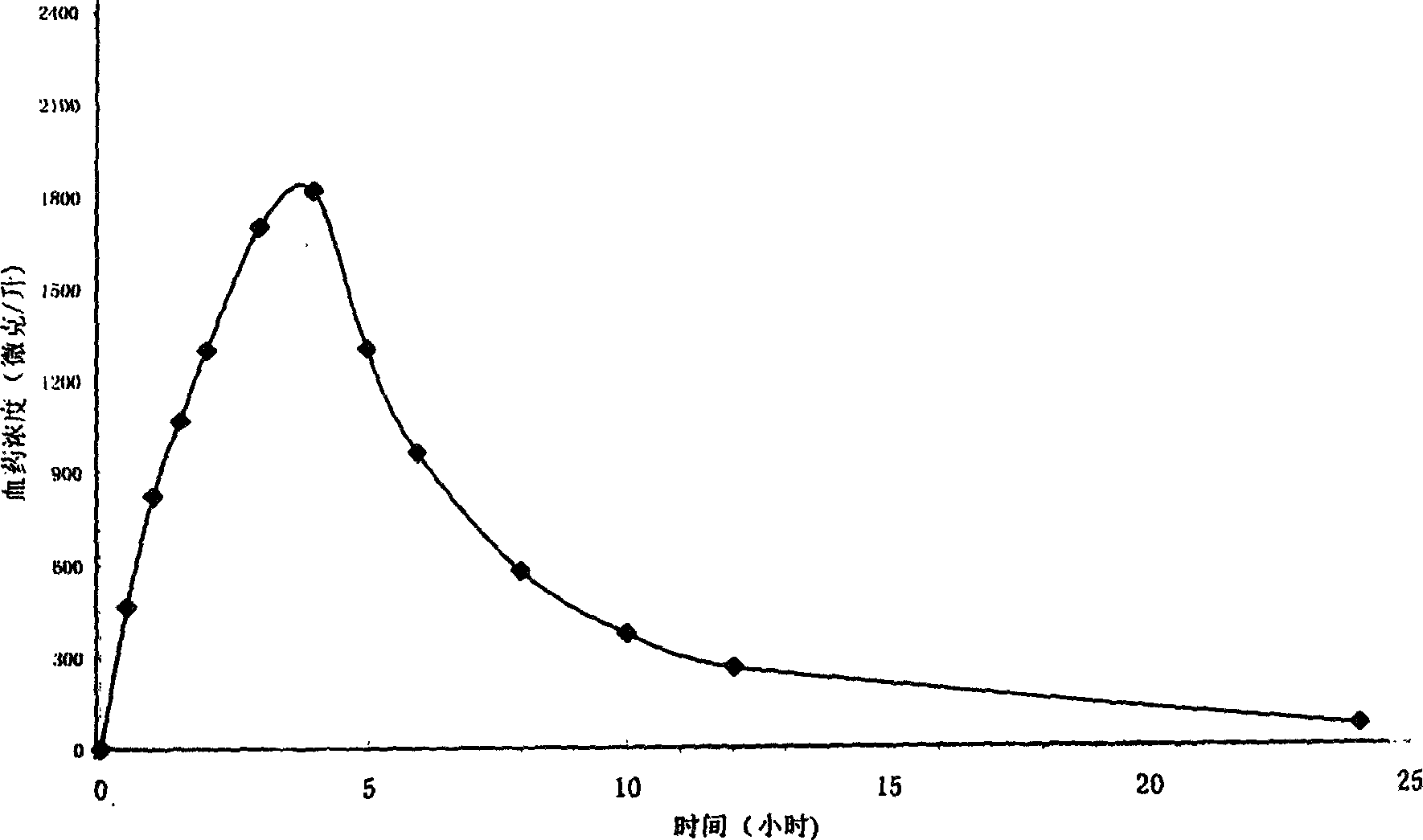
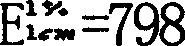Metformin hydrochloride sustained-release tablet and method for preparing the same
A technology of metformin hydrochloride and sustained-release tablets, which is applied in pharmaceutical formulations, medical preparations containing active ingredients, metabolic diseases, etc., can solve problems such as pain and inconvenience for patients, and achieve the advantages of simple process, reduced raw material cost, and reduced dosage. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Take by weighing metformin hydrochloride 500g, hypromellose (model 15KM) 105g; Micropowder ethyl cellulose 110g (model: 10FP, specification: viscosity is 9.0~11.0 centipoise, ethoxyl content is 48.0~49.5%, average The grain diameter is 3-15), ethyl cellulose (model: 10CP) 7g, talcum powder 5g, magnesium stearate 2g.
[0050] Preparation: 500 g of metformin hydrochloride (preliminarily crushed, passed through 100 mesh) and hydroxypropyl methylcellulose (HPMCK15M). Mix mechanically, add 10% ethyl cellulose (85% ethanol) to granulate during stirring, dry and granulate. After the above-mentioned sized dry granules, add micronized ethylcellulose dry powder, talcum powder and magnesium stearate, place in a granulator, and mix evenly. Tablet, get 1000 pieces. Each tablet contains 500mg of metformin hydrochloride, and the weight of the tablet is 729mg.
Embodiment 2
[0052] Take by weighing metformin hydrochloride 500g, hydroxypropyl methylcellulose (model 100KM) 150g; Micropowder ethylcellulose 110g (model: 7FP, specification: viscosity is 6.0~8.0 centipoise, and ethoxyl content is 48.0~49.5%, average The grain diameter is 5-15), ethyl cellulose (type: 10CP) 5g, lactose 30g, talc powder 1g, magnesium stearate 4g.
[0053] Operation method: powder metformin hydrochloride and lactose, pass through 100 mesh, hydroxypropyl methylcellulose pass through 80 mesh, mechanically mix, add 8% ethyl cellulose (70% ethanol) to granulate during stirring, dry, whole grain. After the above-mentioned sized dry granules, add micronized ethylcellulose dry powder, talcum powder and magnesium stearate, place in a granulator, and mix evenly. Tablet, get 1000 pieces. Each tablet contains 500mg of metformin hydrochloride, and the weight of the tablet is 811mg.
Embodiment 3
[0055] Weigh 500 g of metformin hydrochloride, 350 g of hydroxypropyl methylcellulose (model: 4KM); 125 g of micronized ethylcellulose (model: 7FP), 25 g of lactose, 4 g of talc, and 5 g of magnesium stearate.
[0056] Operation method: powder metformin hydrochloride and lactose, pass through 100 mesh, and pass through 100 mesh of hydroxypropyl methylcellulose. Mechanically mix, add 65% ethanol to granulate during stirring, dry and granulate. After the above-mentioned sized dry granules, add micronized ethylcellulose dry powder, talcum powder and magnesium stearate, place in a granulator, and mix evenly. Tablet, get 1000 pieces. Each tablet contains 500mg of metformin hydrochloride, and the weight of the tablet is 1000mg.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com