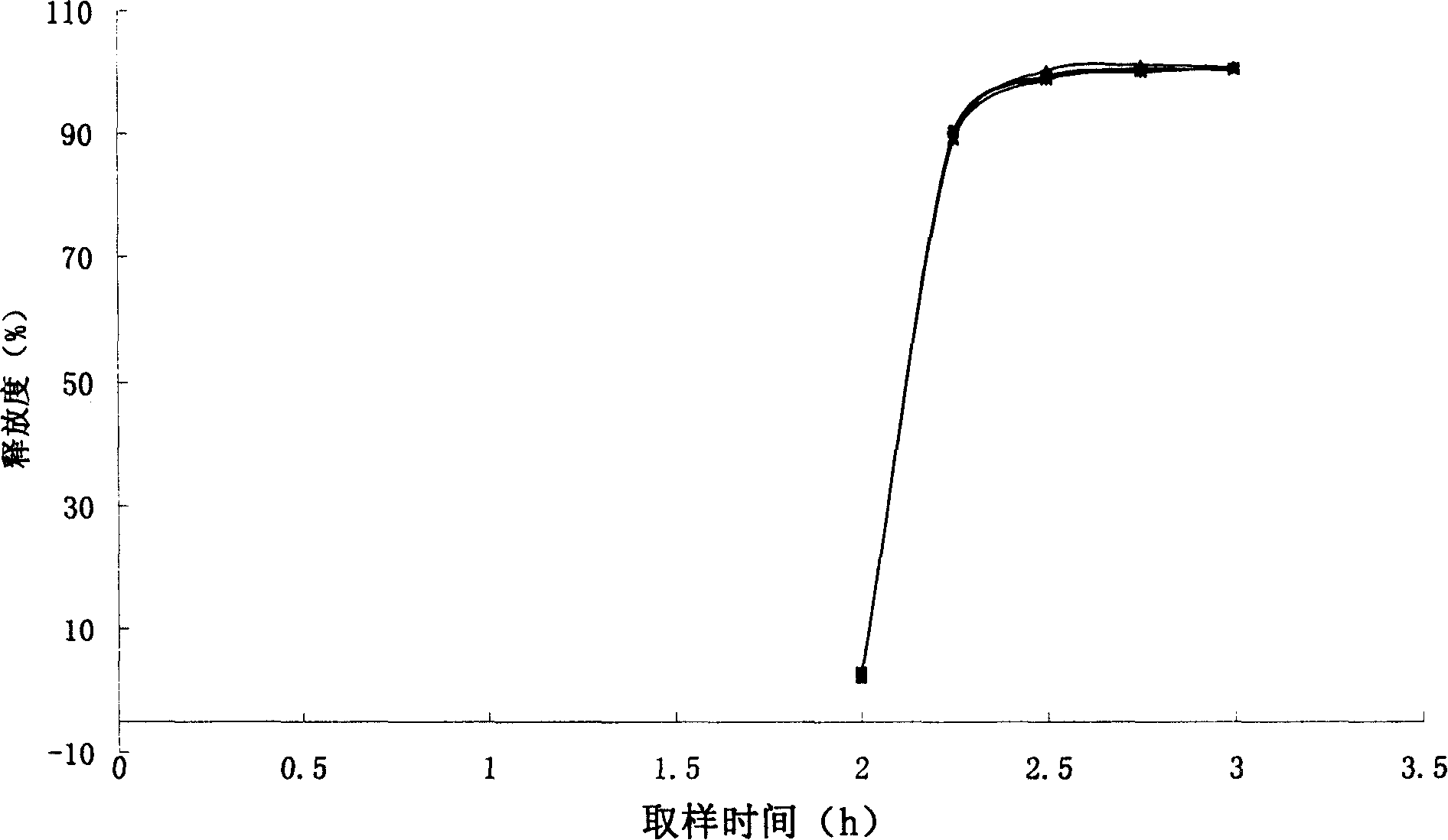
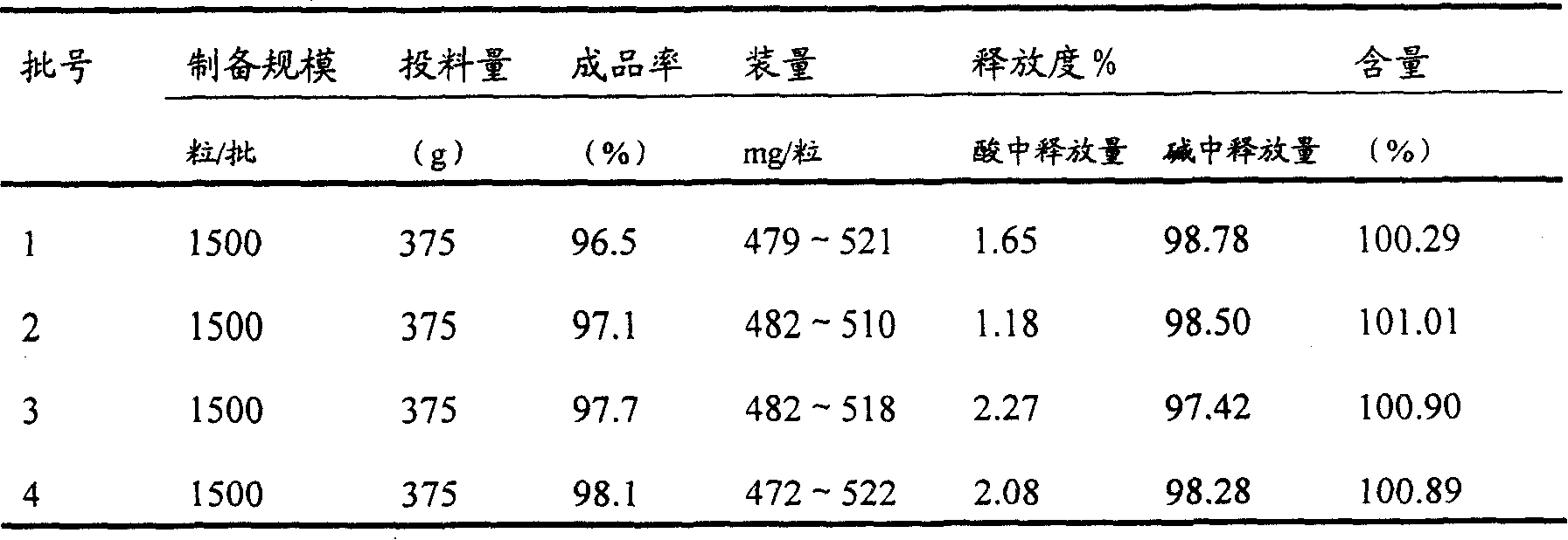
Enteric medicinal composition for treating diabetes and preparation method thereof
A composition and anti-diabetic technology, which can be used in drug combinations, pharmaceutical formulas, sugar-coated pills, etc., can solve the problems of gastric irritation of the main drug, achieve uniform drug release, good quality controllability, and reduce individual differences
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1: the preparation of blank ball core
[0022] Get the microcrystalline cellulose 400g that crosses 80 mesh sieves, place in the centrifugal type one-step granulation coating pot, take 475ml water as binder, set the host speed to be 330r / min, the spray pump speed to be 25r / min, The injection pressure of the spray gun is 0.15MPa, the injection flow rate is 12L / min, and the centrifugal granulation is carried out. After the binder was added, the chassis continued to roll for 1 min to form microcrystalline cellulose core particles. Dry at 60°C for 2 hours.
[0023] Pass the dried microcrystalline cellulose core particles through a 26-mesh sieve, take the core particles under the sieve and pass through a 35-mesh sieve to obtain core particles with a particle size between 610 μm and 750 μm, weigh them, and calculate the average composition. The pellet rate was 61.8% (n=10).
Embodiment 2
[0024] Embodiment 2: ball core spraying medicine
[0025] Metformin Hydrochloride 250g
[0026] Hydroxypropyl Methyl Cellulose 20cps 10g
[0028] water 800ml
[0029] Preparation process: Take 250g of metformin hydrochloride, 5g of hydroxypropyl methylcellulose, add 800ml of water, dissolve with slight heat under stirring, slowly add 25g of talcum powder before coating, and stir to form a suspension. Take 110 g of the blank pellet core prepared in Example 1 and place it in a fluidized bed one-step granulation coating pan. g / min. The above-mentioned medicinal solution is applied to the drug coating under the stirring state, and the drying is continued for 2 minutes after the coating is completed. Weighing, the calculated drug application rate is 94.5%, and the pellet adhesion degree is 3.4%.
[0030] Drug loading rate%=[(total weight of pellets-amount of blank pellet core-amount of binder) / dosage amount]×100%.
[0031] Degree of adhesion = (total...
Embodiment 3
[0032] Embodiment 3: ball core spraying medicine
[0033] Metformin Hydrochloride 250g
[0034] Hydroxypropyl Methyl Cellulose 6cps 10g
[0036] water 800ml
[0037]Preparation process: Take 250g of metformin hydrochloride, 10g of hydroxypropyl methylcellulose, add 800ml of water, dissolve with slight heat under stirring, slowly add 25g of talcum powder before coating, and stir to form a suspension. Take 110g prepared in Example 1 and place it in a fluidized bed one-step granulation coating pot, set the air inlet temperature at 60°C, the air pressure at 0.35bar, the atomization pressure at 1.5bar, and the spray rate at 7.5g / min . The above-mentioned medicinal solution is applied to the drug coating under the stirring state, and the drying is continued for 2 minutes after the coating is completed. Weigh, and calculate drug application rate and degree of adhesion by the method in embodiment 2, the drug application rate is 97.6%, and the degree of ad...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com