Sustained-release pearl clear-sighted eye drops and preparation method thereof
An eye drop, slow-release technology, which is applied in the direction of medical formula, unknown raw materials, medical preparations containing active ingredients, etc., can solve the problems of drug side effects, frequent administration of pearl eye drops, etc., to achieve release The effect of drug uniformity and prolonging the time of drug concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1 Sustained-release pearl eye drops and preparation method thereof
[0032] (1) Prescription: The specific composition of each 1000mL is:
[0033] Pearl liquid 20mL,
[0034] borneol 1.0g,
[0035] Ethylparaben 0.5g,
[0036] Hydroxypropyl Beta Cyclodextrin 8g,
[0037] Propylene glycol 5mL,
[0039] The rest is water for injection.
[0040] (2) Preparation of pearl liquid: Weigh 500g of pearl powder, add it to 2000mL of water for injection, heat to 95-100°C, keep warm for 2 hours, then cool to about 60°C, stop stirring for 1 hour, and separate the supernatant liquid, concentrated to 500mL, and then filtered through a plate-and-frame filter with a 0.45 μm microporous membrane to obtain pearl liquid, and the protein content of the pearl liquid was determined to be 1.5 mg / mL.
[0041] (3) Preparation of slow-release Pearl Eye Drops: ①Add 8.9g of sodium chloride to an appropriate amount of water for injection, heat to boil, ...
Embodiment 2
[0042] Embodiment 2 Sustained-release pearl eye drops and preparation method thereof
[0043] (1) Prescription: The specific composition of each 1000mL is:
[0044] Pearl liquid 20mL,
[0045] borneol 1.0g,
[0046] Ethylparaben 0.5g,
[0047] Hydroxypropyl Beta Cyclodextrin 10g,
[0048] Propylene glycol 3mL,
[0049] Sodium chloride 8.9g,
[0050] The rest is water for injection.
[0051] (2) Preparation of pearl liquid: Weigh 500g of pearl powder, add it to 2000mL of water for injection, heat to 95-100°C, keep warm for 2 hours, then cool to about 60°C, stop stirring for 1 hour, and separate the supernatant liquid, concentrated to 500mL, and then filtered through a plate-and-frame filter with a 0.45 μm microporous membrane to obtain pearl liquid, and the protein content of the pearl liquid was determined to be 1.5 mg / mL.
[0052] (3) Preparation of slow-release Pearl Eye Drops: ①Add 8.9g of sodium chloride to an appropriate amount of water for injection, heat to boil,...
Embodiment 3
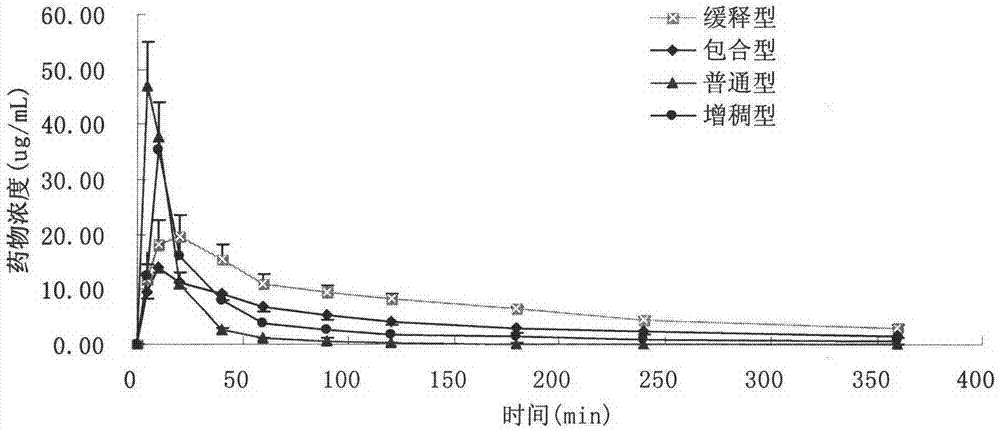
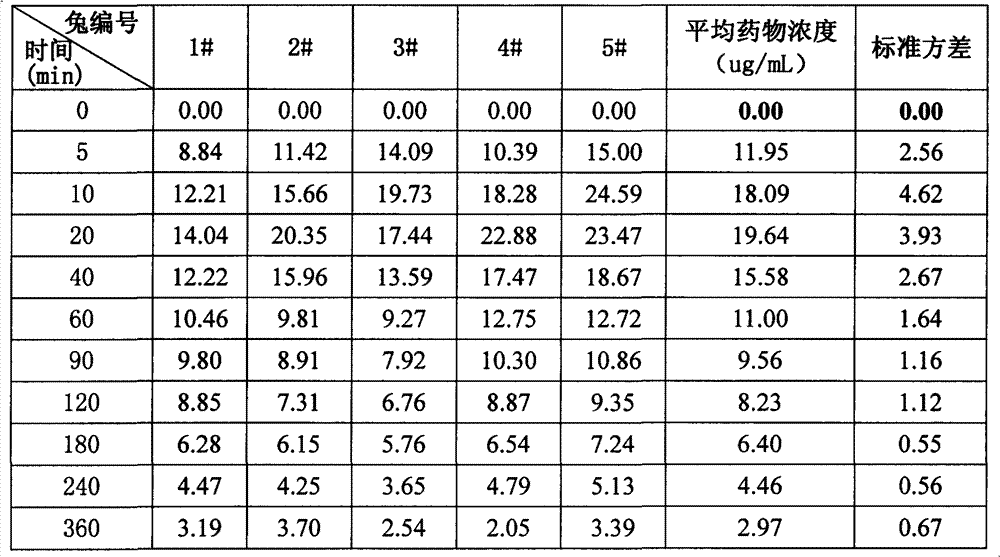
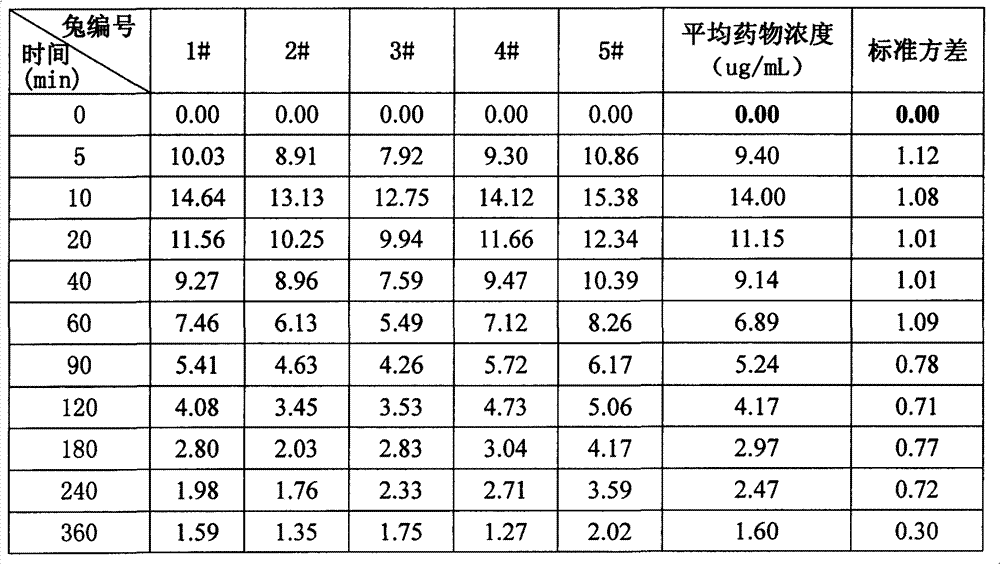
[0053] Example 3 Pharmacokinetic Comparison of Different Types of Pearl Eye Drops
[0054] (1) Purpose of the test
[0055] In this test, taking the representative ingredient borneol in the medicine as an index, the slow-release pearl eye drops sample of the invention embodiment 1, the common pearl eye drops, the thickened pearl eye drops, and different Comparing single-dose pharmacokinetics and drug-release properties of inclusion-type pearl eye drops containing propylene glycol in rabbit tears to investigate their advantages and disadvantages.
[0056] (2) Experimental plan
[0057] drug:
[0058] ① slow-release pearl eye drops of the present invention: prepared according to the above-mentioned embodiment 1;
[0059] ②Inclusion type pearl eye drops: except that propylene glycol is not added, the preparation method is prepared according to Example 1;
[0060] ③ Ordinary pearl eye drops (Suzhou Industrial Park Tianlong Pharmaceutical Co., Ltd., batch number: 110405);
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com