Metformin hydrochloride sustained-release tablet
A technology of metformin hydrochloride and sustained-release tablets, which can be used in medical preparations with non-active ingredients, metabolic diseases, organic active ingredients, etc., can solve problems such as high price, and achieve the effect of low raw material cost and good sustained-release performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
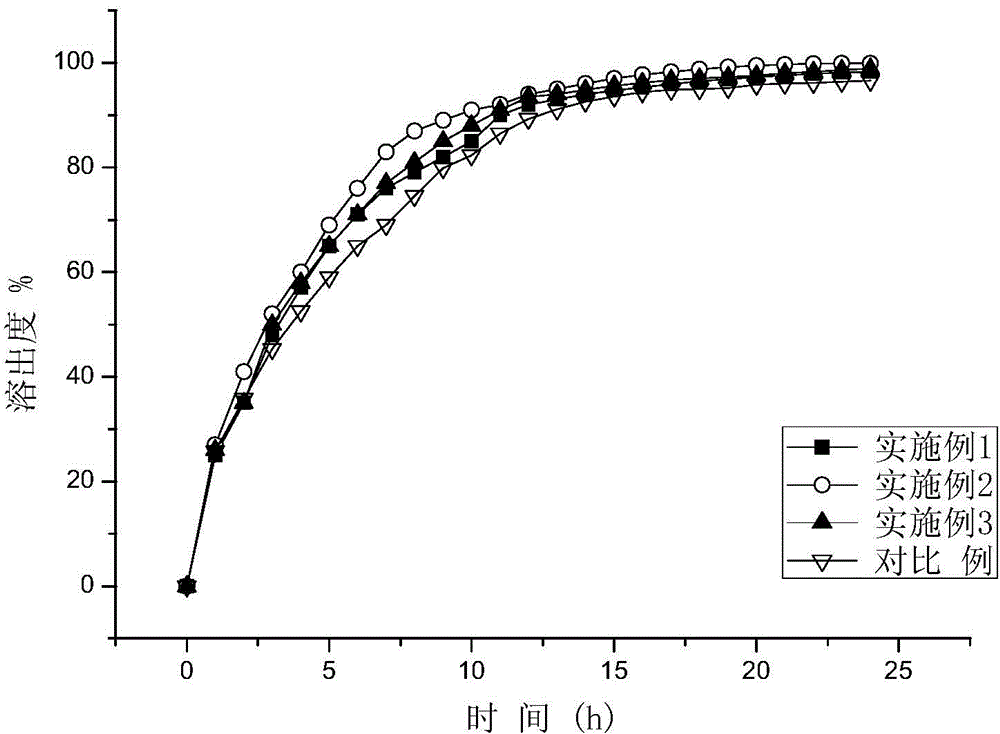
Embodiment 1
[0034] Raw material: Metformin hydrochloride (raw material, calculated as C4H in dry form) 11 N 5 HCl content 98.5-99.0%) 500g, sodium carboxymethylcellulose (viscosity 750-1400mpa s) 45g, hypromellose (Shandong Ruitai 75RT100000) with a viscosity of 100000mpa s at 20°C 160g, at 20°C 78g of hypromellose (Shandong Ruitai 75RT4000) with a viscosity of 4000mpa·s, 191g of ethyl acrylate-methyl methacrylate copolymer aqueous dispersion, and 8g of magnesium stearate.
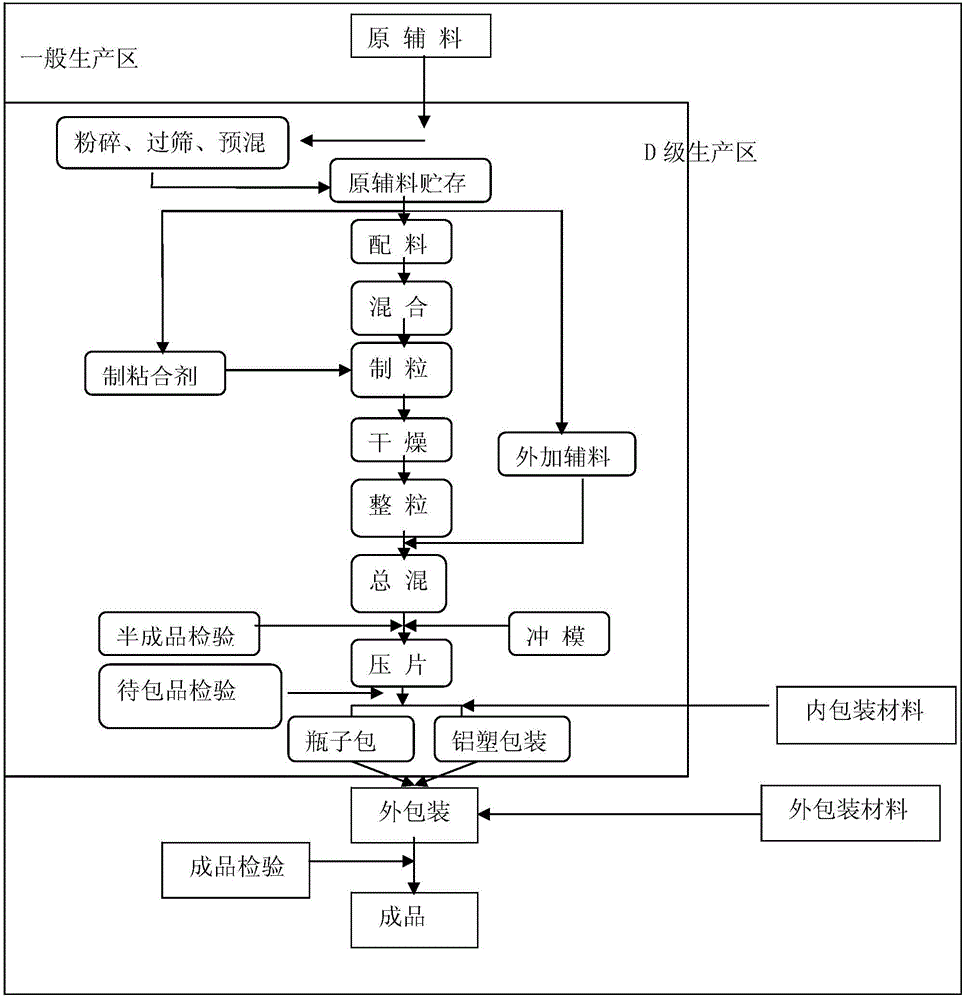
[0035] Plant environment: temperature 18°C-26°C Humidity requirement: 45%-65%, dust-producing rooms (such as crushing, granulation) have a relative negative pressure of 5-8 Pa.
[0036] Among the raw materials, the ethyl acrylate-methyl methacrylate copolymer aqueous dispersion permeates the slow-release material, which is made of the following raw materials in the following weight ratio: ethyl acrylate: 105, methyl methacrylate: 55, purified water: 390, potassium persulfate 0.9, alkylphenol polyoxyethylene ether (O...
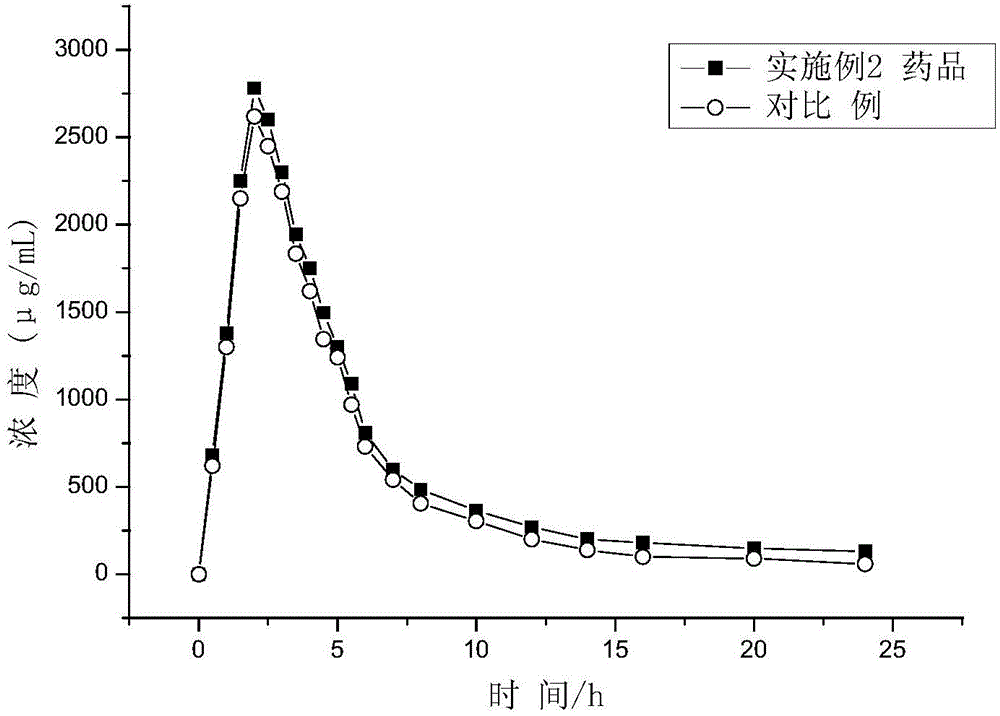
Embodiment 2
[0048] Raw material: Metformin hydrochloride (API, C4H calculated as dry product 11 N 5 ·HCl content 98.5-99.0%) 500g, sodium carboxymethyl cellulose (viscosity 750-1400mpa s) 47g, hypromellose with viscosity 100000mpa s at 20℃ (Shandong Ruitai 75RT100000) 154g, at 20℃ Hypromellose with a viscosity of 4000 mpa·s (Shandong Ruitai 75RT4000) 81 g, ethyl acrylate-methyl methacrylate copolymer aqueous dispersion 193 g, and magnesium stearate 8 g.
[0049] Others are the same as in Example 1.
[0050] The product is 0.5g white film-coated tablet, which appears white after removing the coating. This product + water shake to dissolve metformin, filter, add 10% sodium nitroferricyanide-potassium ferricyanide-10% sodium hydroxide solution to the filtrate, the solution turns red within 3 minutes. According to UV-visible spectrophotometry, it has the maximum absorption at 233nm wavelength.
Embodiment 3
[0052] Raw material: Metformin hydrochloride (API, C4H calculated as dry product 11 N 5 ·HCl content 98.5-99.0%) 500g, sodium carboxymethyl cellulose (viscosity 750-1400mpa s) 43g, 2% aqueous solution, hypromellose with viscosity 100000mpa s at 20°C (Shandong Ruitai 75RT100000) 120g , 80g of hypromellose (Shandong Ruitai 75RT4000) with a viscosity of 4000mpa·s at 20°C, 186g of ethyl acrylate-methyl methacrylate copolymer aqueous dispersion, and 8g of magnesium stearate.
[0053] Others are the same as in Example 1.
[0054] The product is 0.5g white film-coated tablet, which appears white after removing the coating. This product + water shake to dissolve metformin, filter, add 10% sodium nitroferricyanide-potassium ferricyanide-10% sodium hydroxide solution to the filtrate, the solution turns red within 3 minutes. According to UV-visible spectrophotometry, it has the maximum absorption at 233nm wavelength.
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com