Content measuring method of metformin hydrochloride enteric coated tablet
A metformin hydrochloride enteric and metformin hydrochloride technology, which is applied in the field of content determination of metformin hydrochloride enteric-coated tablets, can solve the problems of inaccurate ultraviolet spectrophotometry, inaccurate metformin hydrochloride enteric-coated tablets, etc., and achieves the improvement of accuracy and sensitivity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
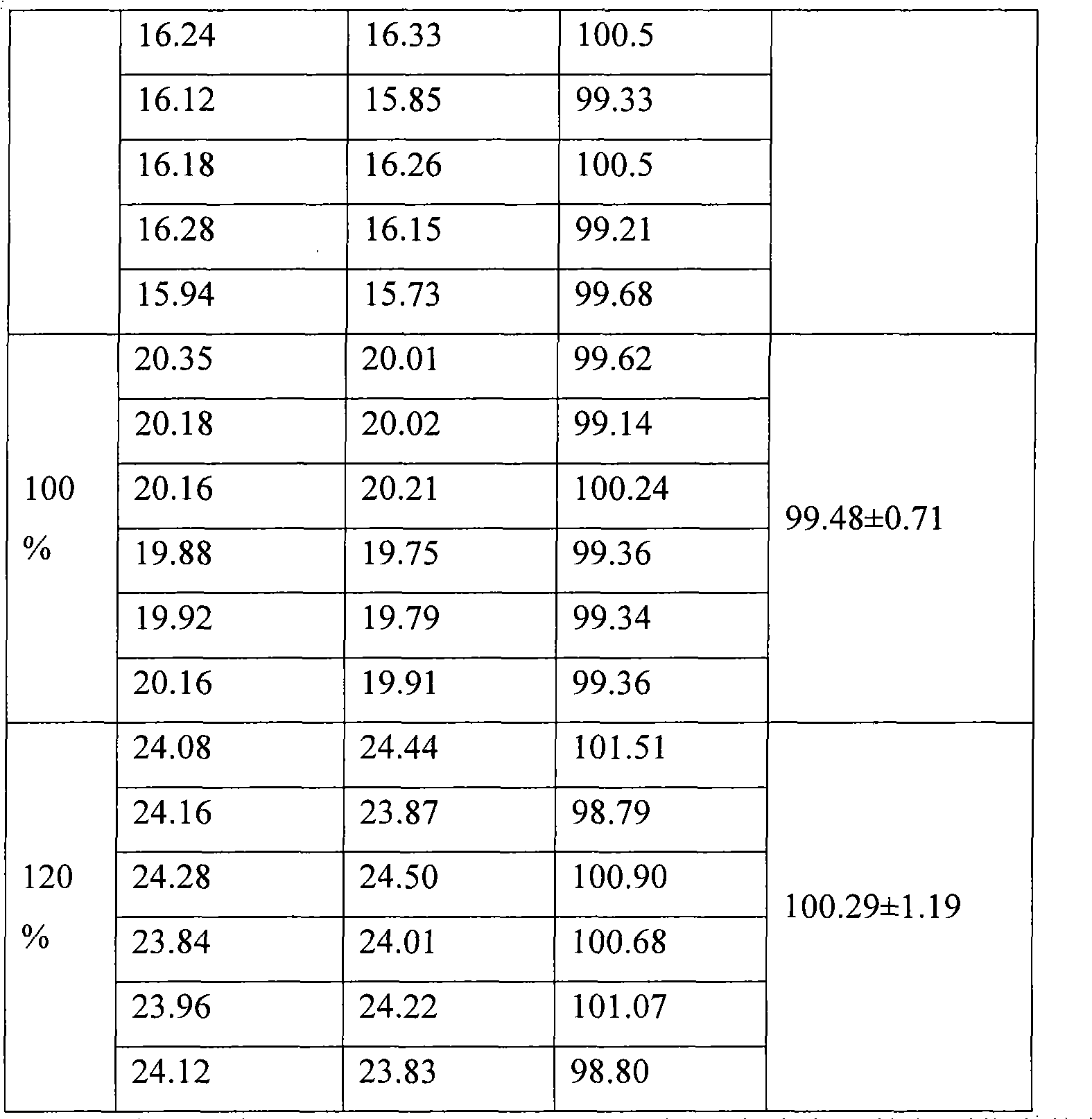
[0064] Chromatographic conditions and system suitability test: use sulfonic acid group cation exchange bonded silica gel as filler, phosphoric acid-1.7% ammonium dihydrogen phosphate solution at pH 3.0:methanol=70:30 (volume ratio) as mobile phase, detect The wavelength is 233nm, and the number of theoretical plates is not less than 3000 based on the metformin hydrochloride peak;
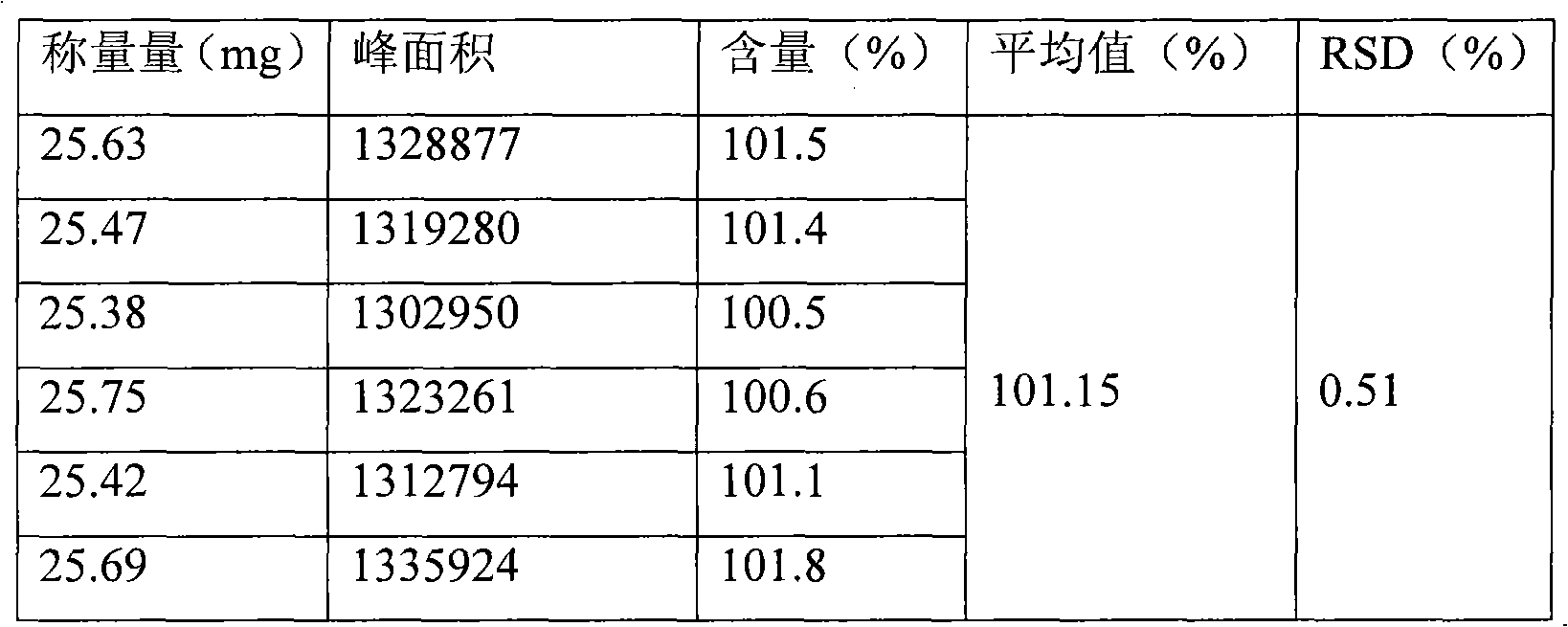
[0065] Preparation of reference substance solution: Take 20 mg of metformin hydrochloride reference substance, accurately weighed, put in a 100ml measuring bottle, add an appropriate amount of mobile phase, sonicate to dissolve metformin hydrochloride and dilute to the mark with mobile phase, shake well, accurately measure 1ml, place In a 10ml measuring bottle, add mobile phase to the mark, shake well, and get it;
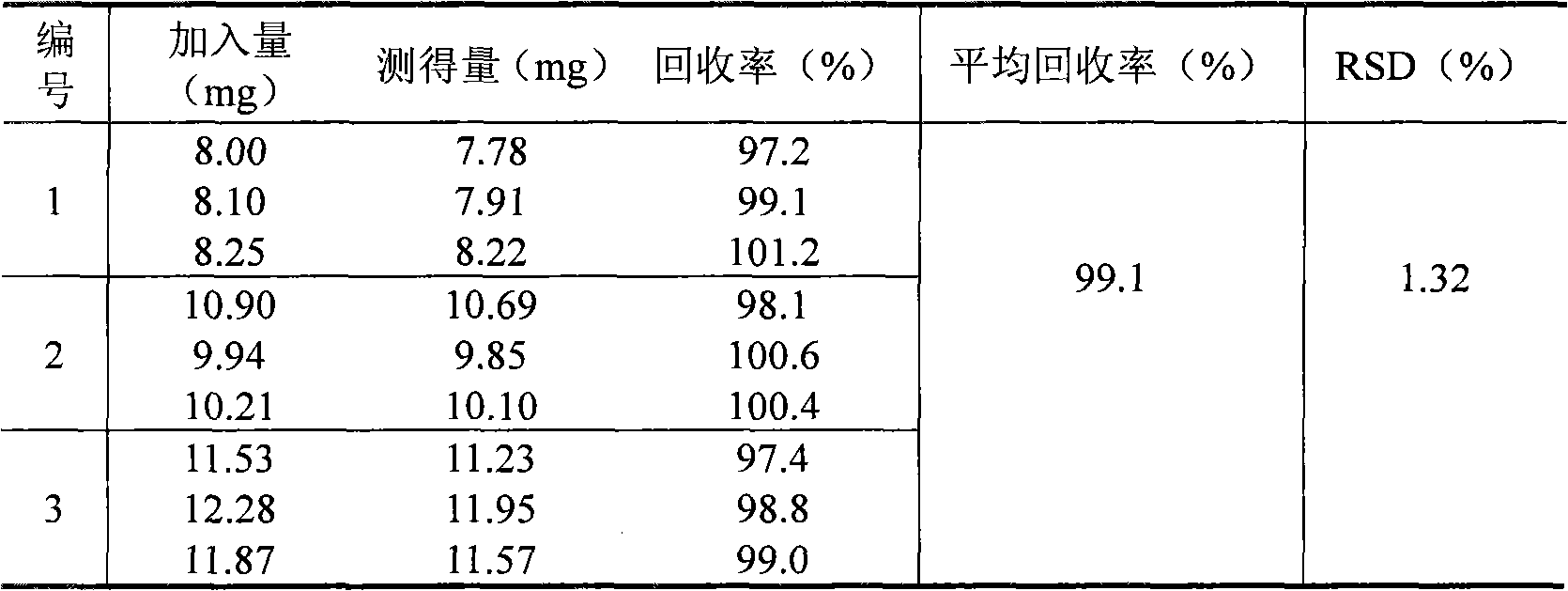
[0066] The preparation of need testing solution: get 10 metformin hydrochloride enteric-coated tablets, grind finely, accurately weigh the appropriate amount of medicine powder (equivale...
Embodiment 2
[0069] Chromatographic conditions and system suitability test: use sulfonic acid group cation exchange bonded silica gel as filler, phosphoric acid-1.7% sodium dihydrogen phosphate solution at pH 2.0: methanol = 90: 10 (volume ratio) as mobile phase, detect The wavelength is 233nm, and the number of theoretical plates is not less than 3000 based on the metformin hydrochloride peak;
[0070] Then measure according to the method of embodiment 1.
Embodiment 3
[0072] Chromatographic conditions and system suitability test: use sulfonic acid group cation exchange bonded silica gel as filler, phosphoric acid-1.7% potassium dihydrogen phosphate solution at pH 4.0:methanol=40:60 (volume ratio) as mobile phase, detect The wavelength is 233nm, and the number of theoretical plates is not less than 3000 based on the metformin hydrochloride peak;
[0073] Then measure according to the method of embodiment 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com