Oral solid drug composition of metformin hydrochloride repaglinide
A technology of metformin hydrochloride and its composition, which is applied in the field of oral solid pharmaceutical composition containing metformin hydrochloride repaglinide and its preparation, and can solve problems such as complex preparation process and difficulty in industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
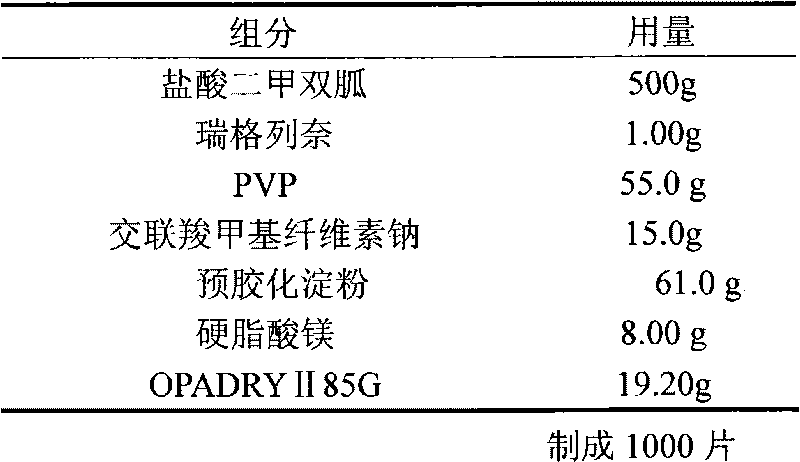
Embodiment 1
[0018]
[0019] Preparation:
[0020] Mix the prescribed amount of metformin hydrochloride, precrossed starch, and PVP evenly, add the ethanol solution of repaglinide, stir evenly, add an appropriate amount of purified water to make a soft material, and granulate with 16 mesh; ventilate and dry at 60°C until the water content is 2-3 %, 24 mesh granules, add croscarmellose sodium and magnesium stearate, mix evenly, press into tablets to make plain tablets, make OPADRY II 85G into a 15% aqueous solution, and coat the weight gain to 3 %, that is.
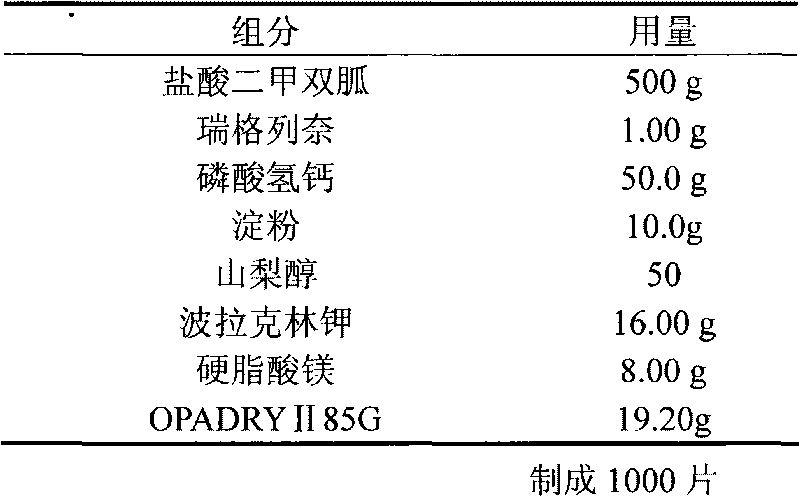
Embodiment 2
[0022]
[0023] Preparation:
[0024] Weigh the prescribed amount of starch, prepare it into 10% starch slurry, and set aside; mix the prescribed amount of metformin hydrochloride and calcium hydrogen phosphate evenly, add the ethanol solution of repaglinide, stir evenly, add starch slurry to make soft material, 16 mesh granules; ventilated and dried at 60°C until the water content is 2-3%, granulated with 24 meshes, added with sorbitol, polacrilin potassium, and magnesium stearate, mixed evenly, pressed into tablets to obtain plain tablets, and OPADRY II 85G was formulated into 15 % aqueous solution, coating weight increase to 3%, that is.
Embodiment 3
[0026]
[0027] Preparation:
[0028] Weigh the prescription amount of HPMC, prepare it into a 2% solution, and set aside; mix the prescription amount of metformin hydrochloride, mannitol, and PVP evenly, add the ethanol solution of repaglinide, after stirring evenly, add the HPMC solution to make a soft material, 16 mesh Granules; ventilated and dried at 60°C until the water content is 2-3%, granulate with 24 mesh, add sodium carboxymethyl starch and magnesium stearate, mix well, press into tablets to obtain plain tablets, and make OPADRY II 85G into a 15% aqueous solution , the weight of the coating is increased to 3%, that is to say.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com