Fuscoporia obliqua active ingredients capable of lowering blood sugar and preparation method and application of fuscoporia obliqua active ingredients
A technology of Inonotus obliquus and Inonotus obliquus, which is applied to the effective hypoglycemic part of Inonotus obliquus and its preparation and application field, can solve the problems of enrichment and separation of active substances, reduce adverse side effects, and have market competitiveness Advantages, the effect of reducing the toxicity of clinical medication
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1, the extraction and separation of the active polysaccharide of Inonotus obliquus fruiting bodies
[0021] (1) After pulverizing the fruiting bodies of Inonotus obliquus, pass through a 40-mesh sieve.
[0022] (2) Preparation of crude polysaccharide extracted at normal temperature: add 30 times the volume of water to the pulverized and sieved Inonotus obliquus, let it stand for extraction for 48 hours, centrifuge the extract (4000r / min), leave the residue, collect the supernatant, Concentrate under reduced pressure to 1 / 10 of the original volume, add absolute ethanol until the final concentration of ethanol is 80%, let it stand overnight at 4°C, collect the precipitate by centrifugation, dry and weigh at 50°C, and obtain crude polysaccharide extracted at room temperature. The polysaccharide content was determined by the phenol-sulfuric acid method, and the calculated polysaccharide yield (polysaccharide yield=(crude polysaccharide weight / material weight)×100%) ...
Embodiment 2
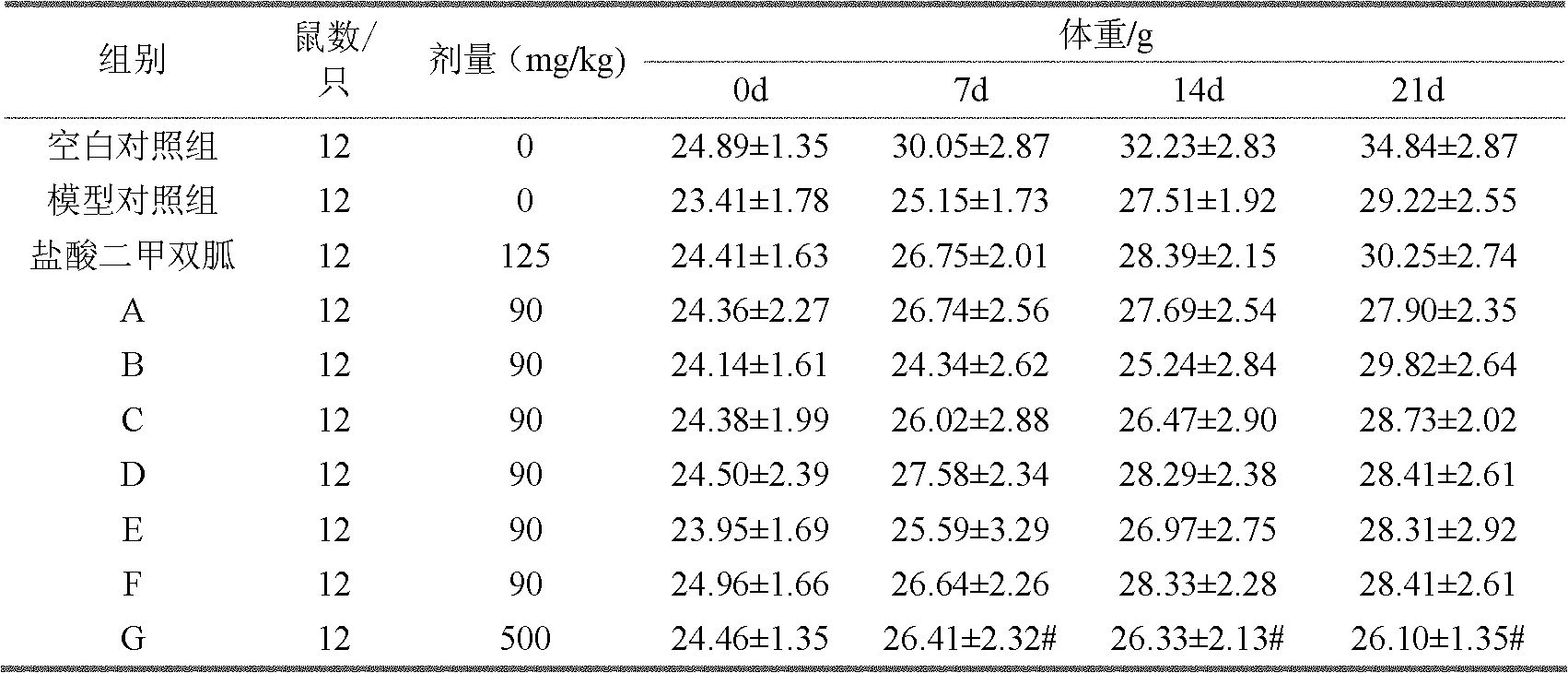
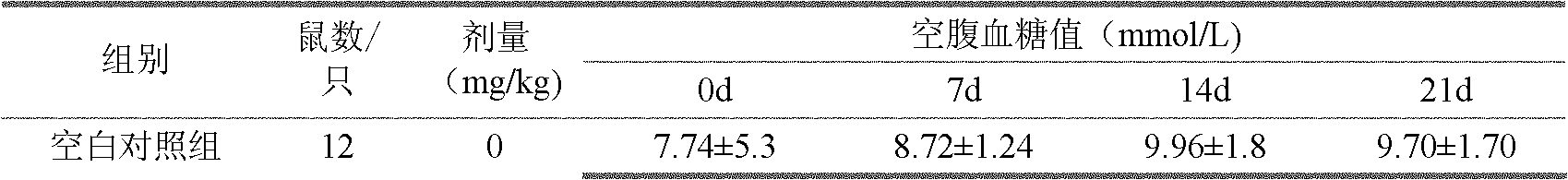
[0027] Example 2, Evaluation of hypoglycemic pharmacological activity of each polysaccharide component of Inonotus obliquus
[0028] (1) Test material:
[0029] The crude extract of Inonotus obliquus and each polysaccharide component of Inonotus obliquus were prepared according to the method in Example 1. Positive control drug: metformin hydrochloride tablets, Beijing Pharmaceutical Co., Ltd., specification: 0.25g×50 tablets / box. Streptozotocin (STZ): American sigma company. Glucose oxidase kit: Beijing Jiuqiang Biotechnology Co., Ltd. Triglyceride kit: Beijing Beihua Kangtai Clinical Kit Co., Ltd. Enzymatic Total Cholesterol Determination Kit: Zhongsheng Beikong Biotechnology Co., Ltd. Insulin radioimmunoassay kit: China Institute of Atomic Energy.
[0030] Test animals: SPF grade ICR mice, male, weighing 18-22g. Provided by the Experimental Animal Center of the Academy of Military Sciences. The animal license number is SCXK (Army) 2002-001.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com