Metformin hydrochloride sustained-release tablets and preparation method thereof
A technology of metformin hydrochloride and sustained-release tablets, which is applied in pharmaceutical formulations, medical preparations with inactive ingredients, and medical preparations containing active ingredients, etc., can solve problems such as easy to forget to take, missed doses, and large fluctuations in blood drug concentration. , to achieve the effect of convenient administration, stable blood concentration and prolonged half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] 0.5% hypromellose solution and 10% magnesium stearate solution were prepared with 70%, 75%, and 95% ethanol respectively;
[0023] Weigh 500 mg of metformin hydrochloride, 175 mg of hypromellose, and 175 mg of sodium carboxymethyl cellulose, mix and sieve. Add the prepared hypromellose pulp, then add the magnesium stearate pulp, granulate and dry.
[0024] Ethanol concentration (%)
Embodiment 2
[0026] The type of hypromellose has a relatively large impact on the sustained release effect. In this example, hypromellose-50, hypromellose-1000 and hypromellose-4000 are used as binders . Analysis of several common models, the results are as follows:
[0027] components
[0028] The results show that: HPMC-4000 can release the drug more slowly than other hypromellose models.
Embodiment 3
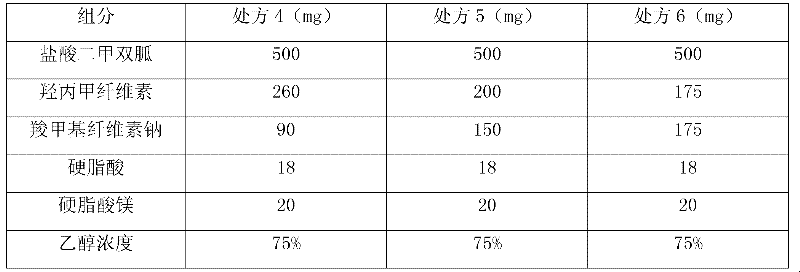
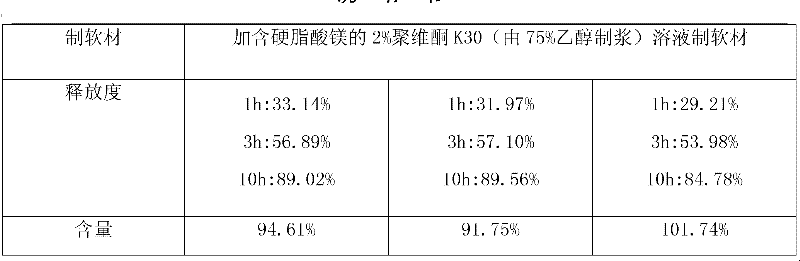
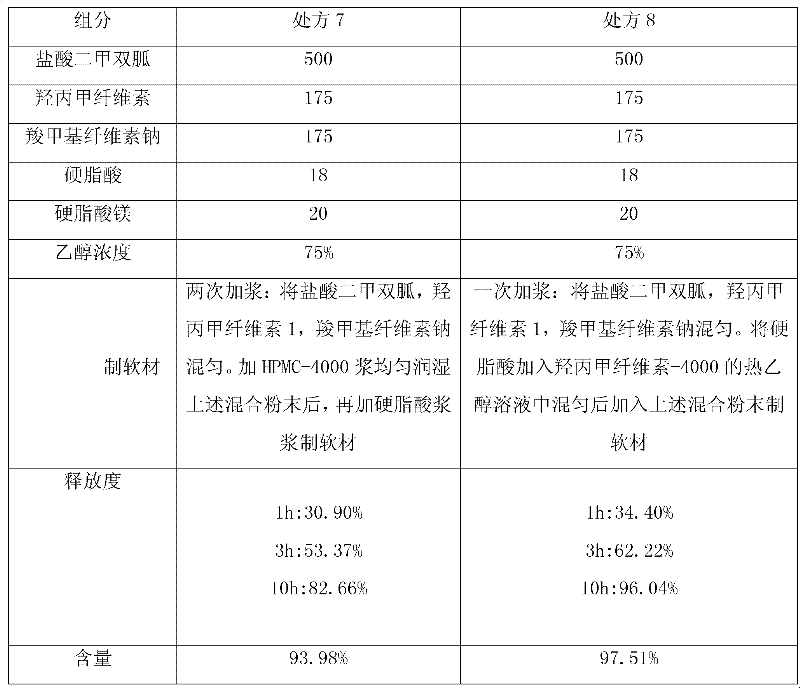
[0030] In order to consider the sustained-release effect of the key excipients hypromellose and sodium carboxymethylcellulose on the tablet, the applicant conducted research on the addition ratio. When making soft materials at one time, select povidone K30 to replace hypromellose pulping to increase the viscosity, and reflect the slow release effect through the tablet release rate. The preferred ratio of hypromellose to sodium carboxymethylcellulose is 1: 1.
[0031]
[0032]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com