Method for producing extended release tablet
A manufacturing method and sustained-release technology, which are applied in the field of sustained-release tablet manufacturing to achieve the effects of efficient manufacturing and improved fluidity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] (Example 1, Comparative Example 1)
[0080] The following components (1) to (4) were mixed in the proportions (parts by weight) shown in Table 1.
[0081] (1) As an active ingredient, commercially available acetaminophen (manufactured by API Corporation) was used (the same applies hereinafter).
[0082] (2) As the excipient component of the additive, DCL (trade name: Pharmatose DCL-21, manufactured by DMV international) was used (the same applies hereinafter).
[0083] (3) As the hydroxyalkyl cellulose (A), use hydroxypropyl cellulose (trade name: HPC-L (fine powder), manufactured by Nippon Soda Co., Ltd.) with a viscosity of 8.1 mPa·s at 20° C. in a 2 mass % aqueous solution. More than 99% of the particles have a particle size of 150 μm or less. Hereinafter abbreviated as “HPC-1”) (the same below).
[0084] (4) In addition, as the hydroxyalkylcellulose (B) having a viscosity of 100 mPa·s or more at 20° C. in a 2 mass % aqueous solution, hydroxypropyl cellulose having...
Embodiment 2、3
[0090] (embodiment 2,3, comparative example 2,3)
[0091] The following components (1) to (4) were mixed in proportions (parts by weight) shown in Table 2.
[0092] (1) Active ingredient: Acetaminophen
[0093] (2) Excipient: DCL
[0094] (3) HPC-1
[0095] (4)HPC-2
[0096] Table 2
[0097] Table 2
[0098] Example 2 Example 3 Comparative example 2 Comparative example 3 Acetaminophen 3 3 3 3 DCL 47 27 67 47 HPC—1 40 50 — — HPC-2 10 20 30 50
[0099] Next, 300 mg of the mixture obtained above was directly compressed into tablets under a pressure of 10 kN using a hydraulic pressure forming machine (RIKENPOWER, manufactured by RIKEN POWER Co., Ltd.), to obtain flat-top tablets (about 3.2 mm in thickness) with a diameter of 10 mm, respectively. mm).
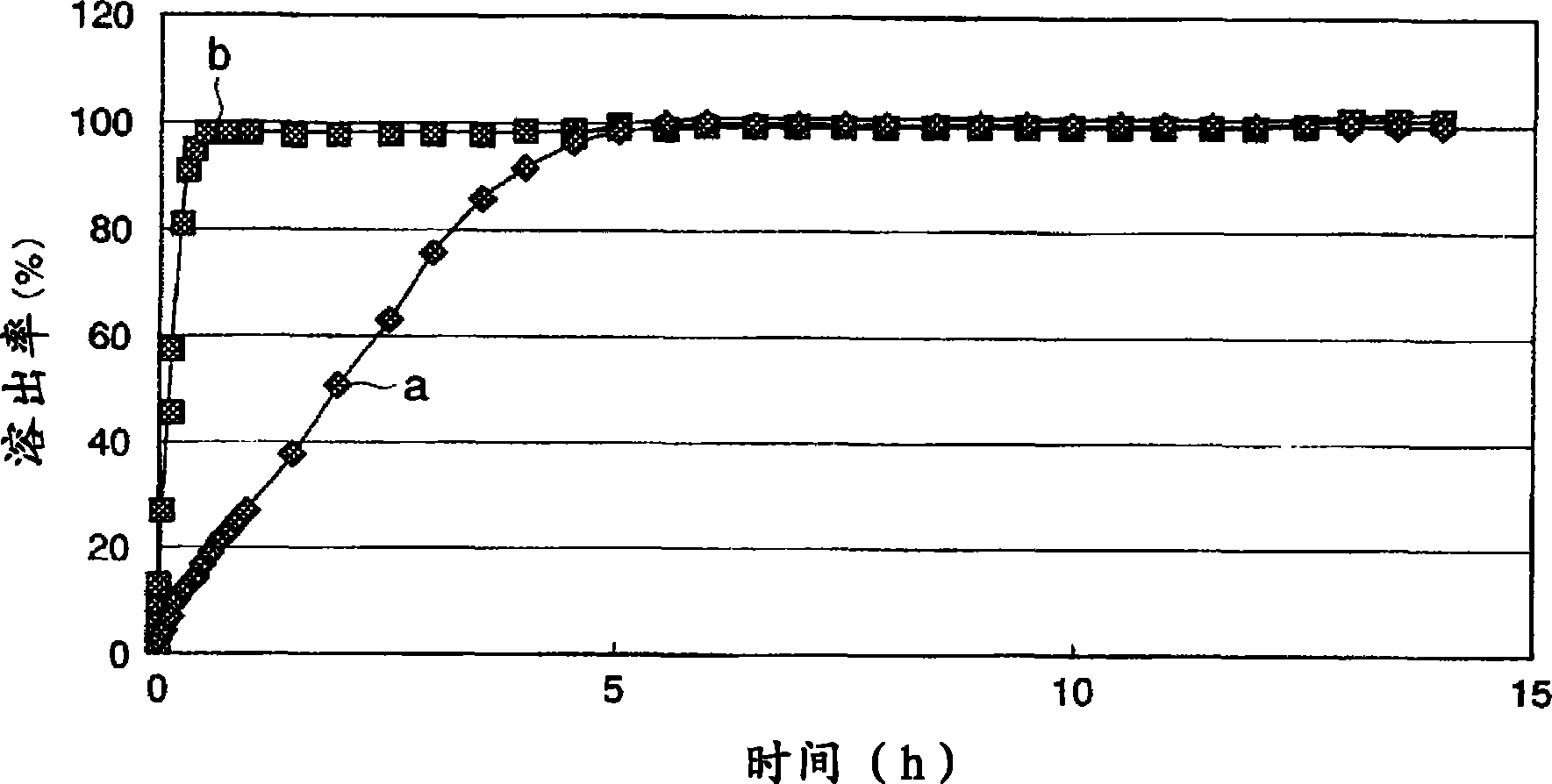
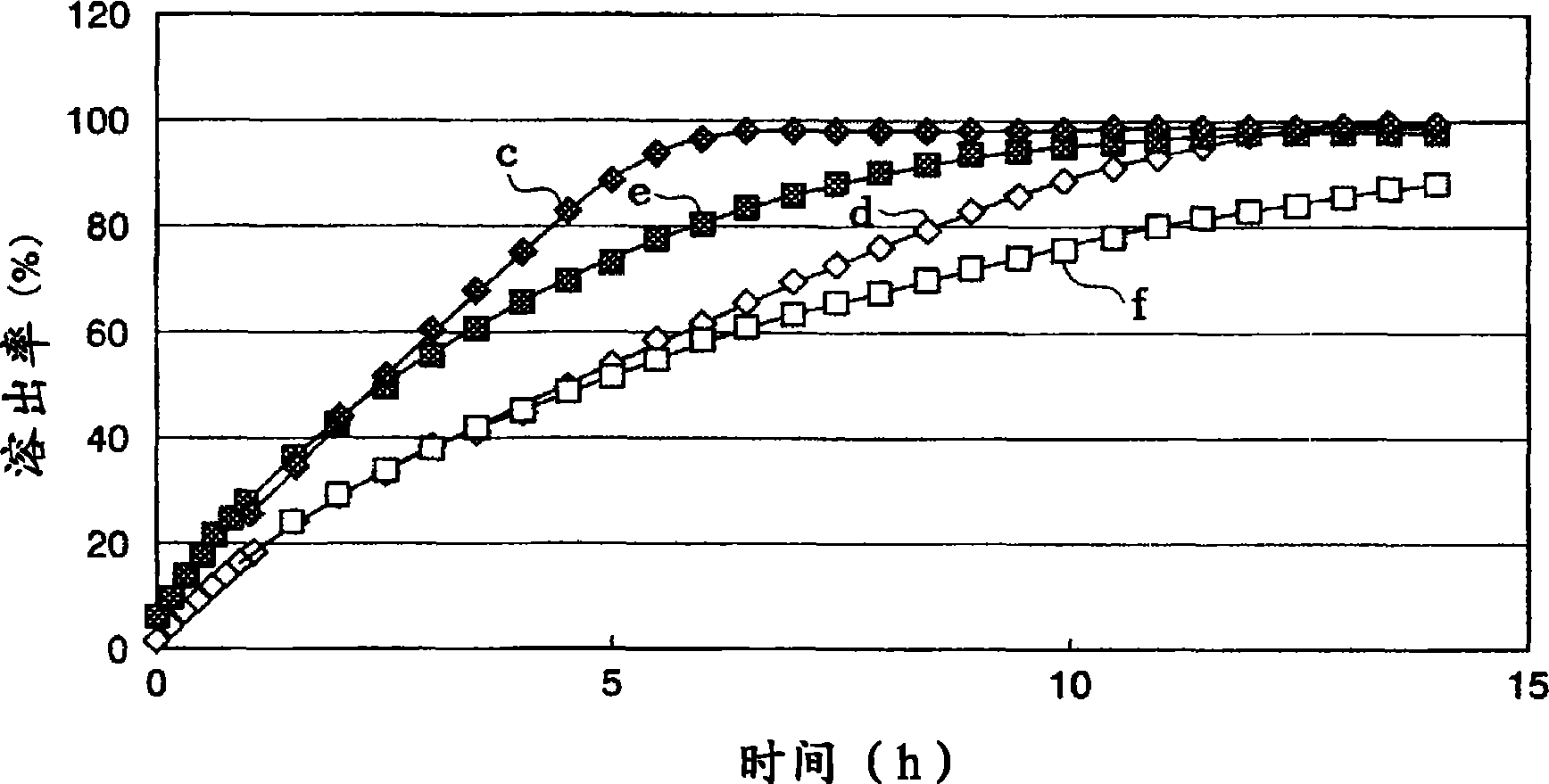
[0100] The lapse of time in water and the dissolution rate (%) of the active ingredient of the tablets obtained in Examples 2 and 3 and Comparative Examples 2 and 3 we...
Embodiment 4~6
[0103] The following components (1) to (4) were mixed in proportions (parts by weight) shown in Table 3.
[0104] (1) Active ingredient: Acetaminophen
[0105] (2) Excipient: DCL
[0106] (3) HPC-1
[0107] (4)HPC-2
[0108] table 3
[0109] table 3
[0110] Example 4 Example 5 Example 6 Acetaminophen 3 3 3 DCL 17 7 7 HPC—1 40 50 40 HPC-2 40 40 50
[0111] Next, 300 mg of the mixture obtained above was directly compressed into tablets using a hydraulic press molding machine (RIKENPOWER, manufactured by Riken Machine Co., Ltd.) under a pressure of 10 kN to obtain flat-topped tablets (about 3.5 mm thick) with a diameter of 10 mm. ).
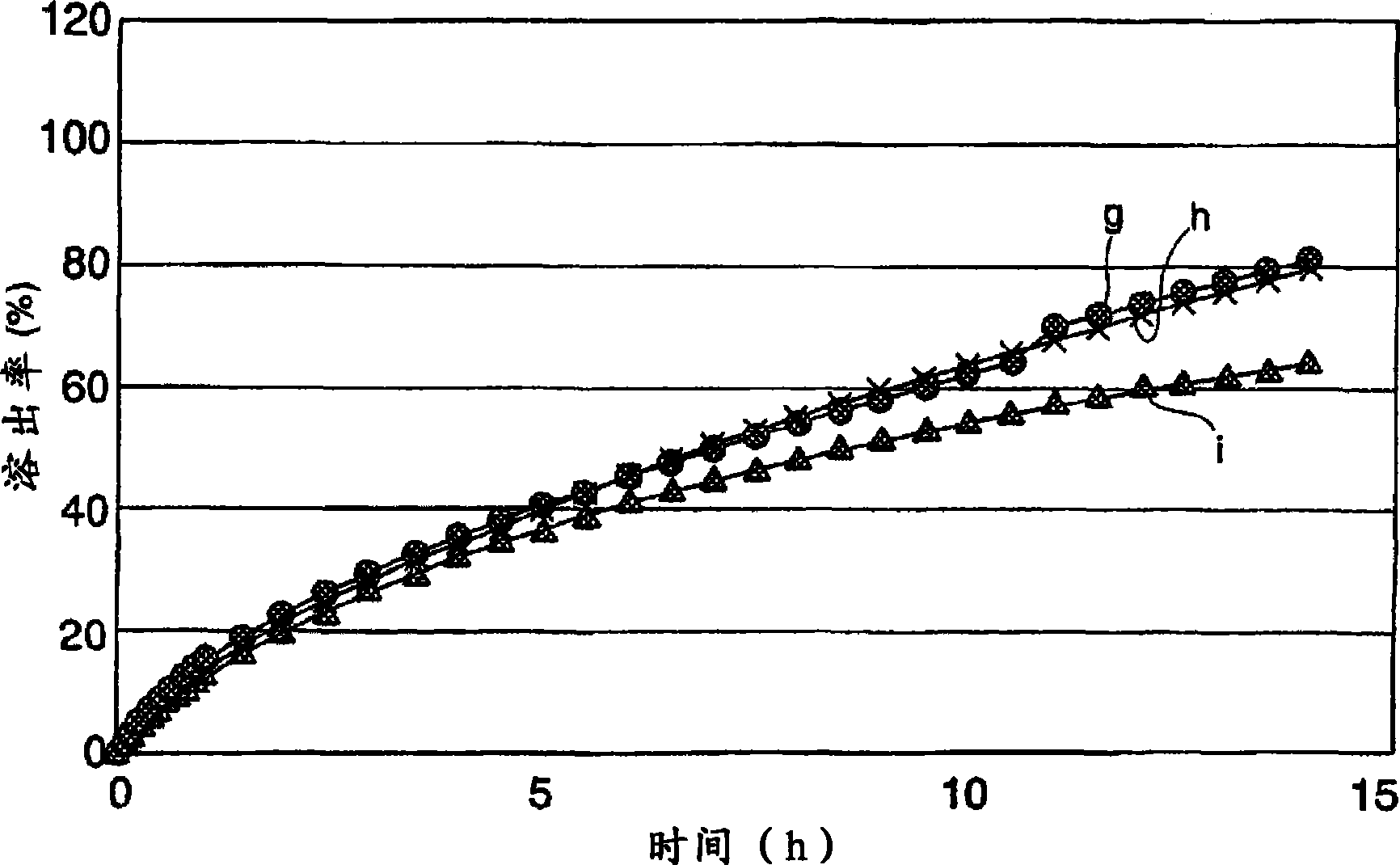
[0112] The lapse of time in water and the dissolution rate (%) of the active ingredient of the tablets obtained in Examples 4 to 6 were measured. The measurement results are shown in image 3 middle. exist image 3 Among them, g is the measurement result of Example 4, h is the measuremen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com