Metformin hydrochloride floating sustained-release tablet and preparation method thereof
A metformin hydrochloride, gastric flotation technology, applied in pharmaceutical formulations, medical preparations with non-active ingredients, and medical preparations containing active ingredients, etc. problem, to achieve the effect of excellent flotation performance and sustained release performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] In the present embodiment, the tablet core prescription of metformin hydrochloride gastric floating sustained-release tablet is as shown in Table 1:
[0046] Table 1
[0047] Element
Weight (mg)
Proportion(%)
1000
80.00
Hypromellose E5
50
4.00
Crospovidone
190
15.20
10
0.80
[0048] The coating prescription outside the tablet core is as shown in Table 2:
[0049] Table 2
[0050] Element
Weight (mg)
Udrake RL100
50
Udrake RS100
50
talcum powder
50
10
95% ethanol
950
[0051] The preparation method is as follows: granulate metformin hydrochloride and hypromellose solution, dry, granulate, mix with crospovidone and glyceryl behenate, and compress into tablets so that the tablet core density is less than 1.0g / cm 3 , coating.
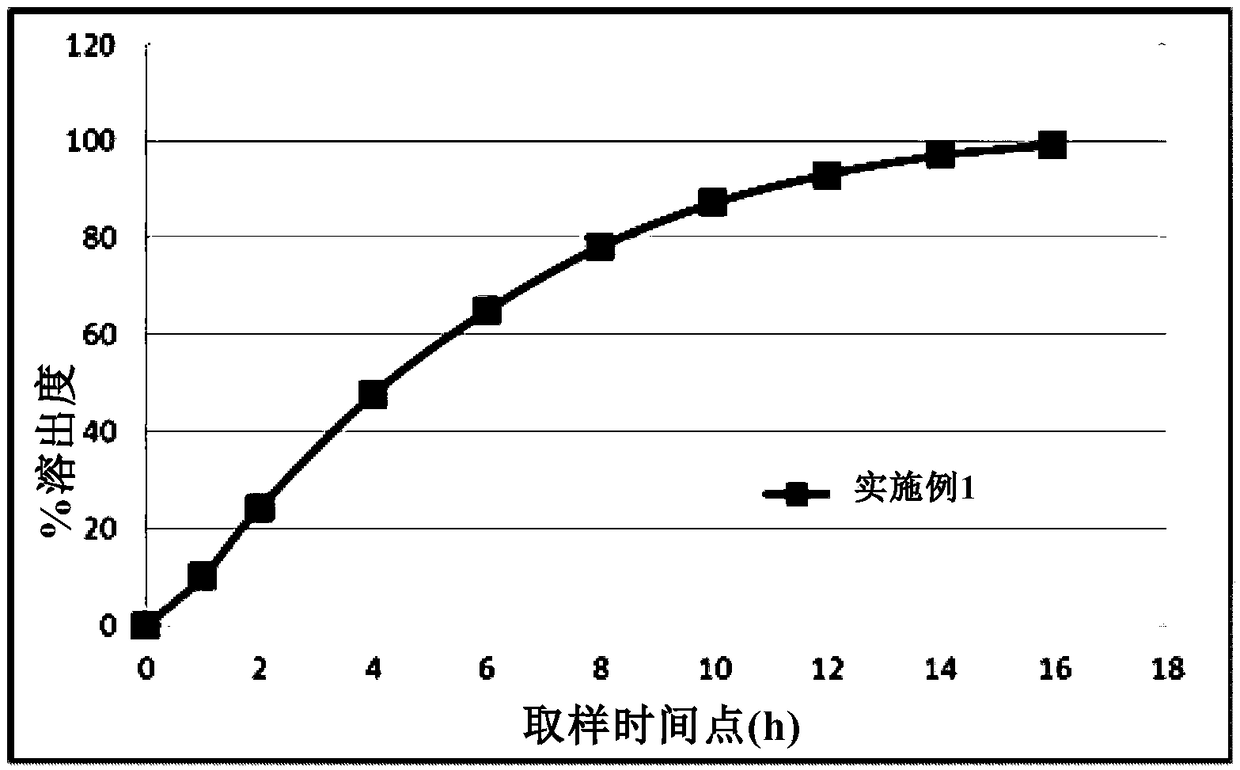
[0052] Metformin hydrochloride gastric floati...
Embodiment 2
[0054] In this embodiment, the tablet core prescription of metformin hydrochloride gastric floating sustained-release tablet is as shown in Table 3:
[0055] table 3
[0056]
[0057]
[0058] The coating prescription outside the tablet core is as shown in Table 4:
[0059] Table 4
[0060] Element
Weight (mg)
Eudragit NE 30D
100
Titanium dioxide
0.9
talcum powder
30
Hypromellose E5
9
170
[0061] The preparation method is as follows: granulate metformin hydrochloride and hydroxypropyl cellulose solution, dry, granulate, mix with crospovidone and glyceryl behenate, and compress into tablets so that the tablet core density is less than 1.0g / cm 3 , coating.
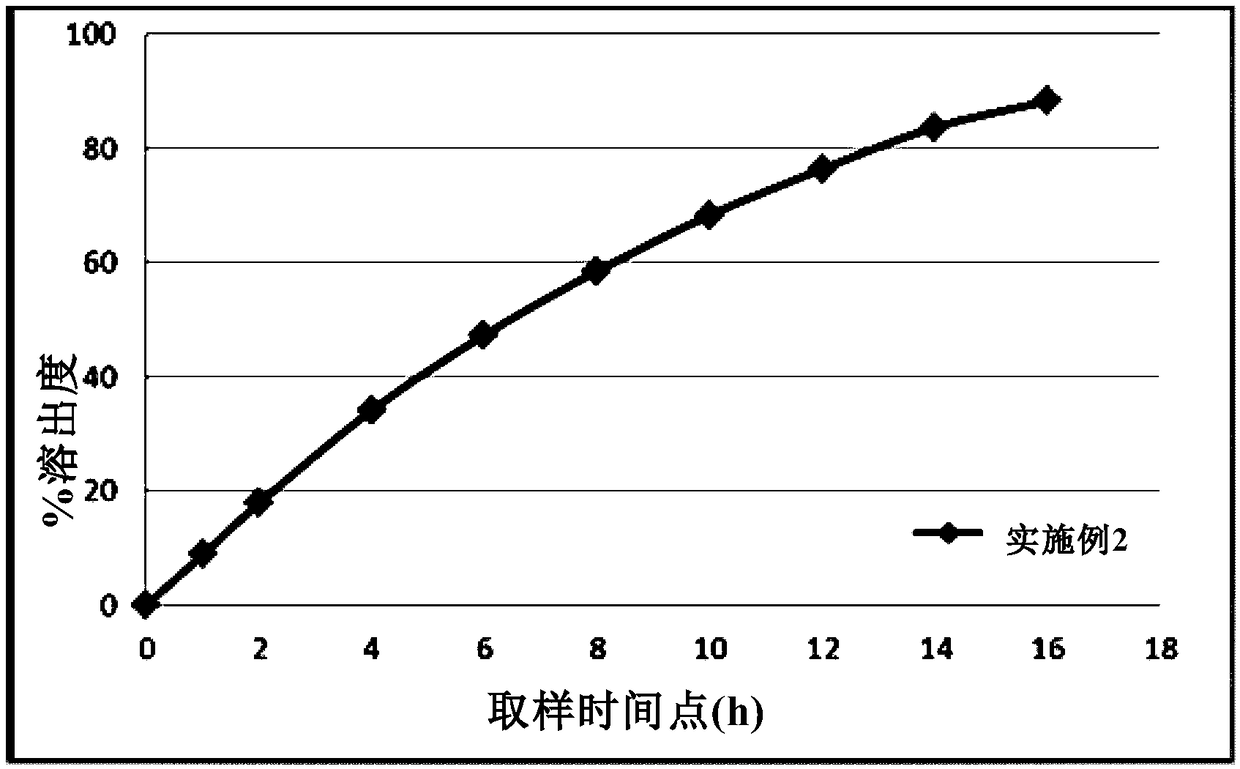
[0062] The dissolution profile of Metformin Hydrochloride Gastric Float Sustained-release Tablet under the same dissolution profile determination condition as described in Example 1 is as follows figure 2 shown.
Embodiment 3
[0064] In the present embodiment, the tablet core prescription of metformin hydrochloride gastric floating sustained-release tablet is as shown in Table 5:
[0065] table 5
[0066]
[0067]
[0068] The coating prescription outside the tablet core is as shown in Table 6:
[0069] Table 6
[0070] Element
Weight (mg)
70
polyethylene glycol
30
90% ethanol
900
[0071] The preparation method is as follows: granulate metformin hydrochloride and polyvinyl alcohol solution, dry, granulate, mix with crospovidone, silicon dioxide, and glyceryl behenate, and compress into tablets so that the tablet core density is less than 1.0 g / cm 3 , coating.
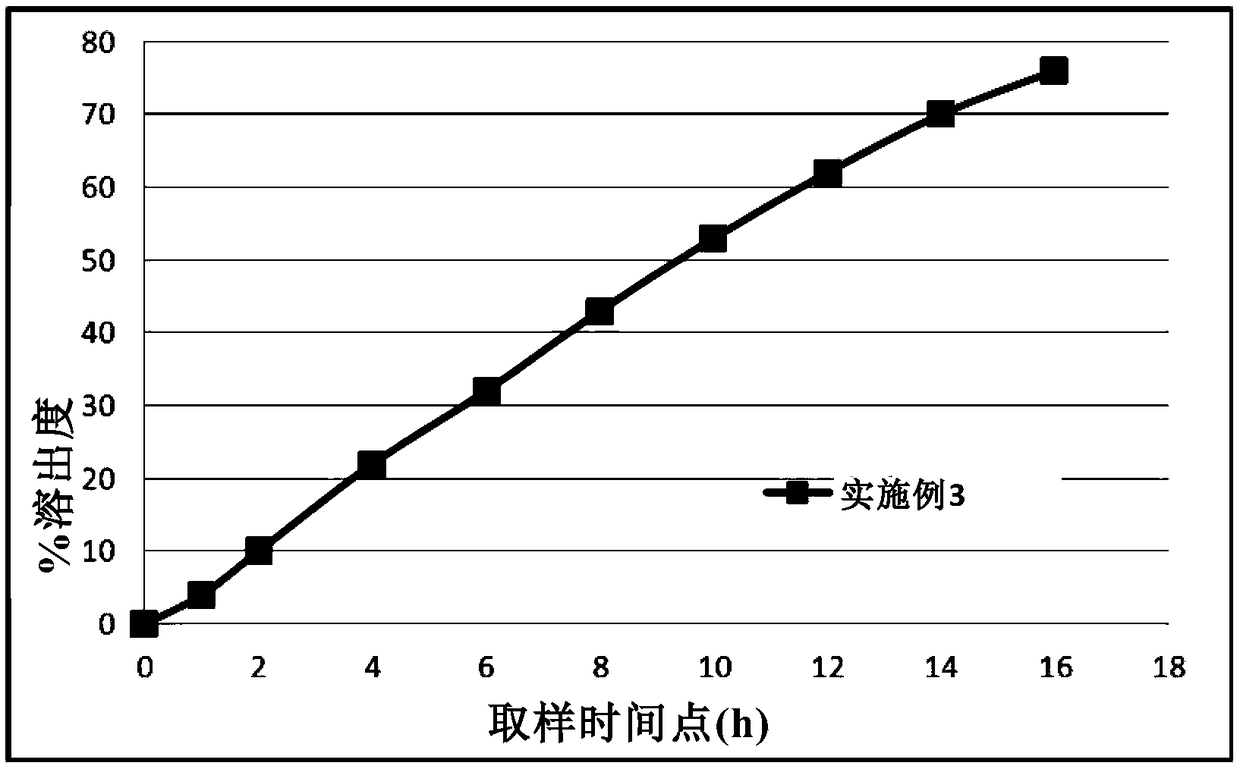
[0072] The dissolution profile of Metformin Hydrochloride Gastric Float Sustained-release Tablet under the same dissolution profile determination condition as described in Example 1 is as follows image 3 shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com