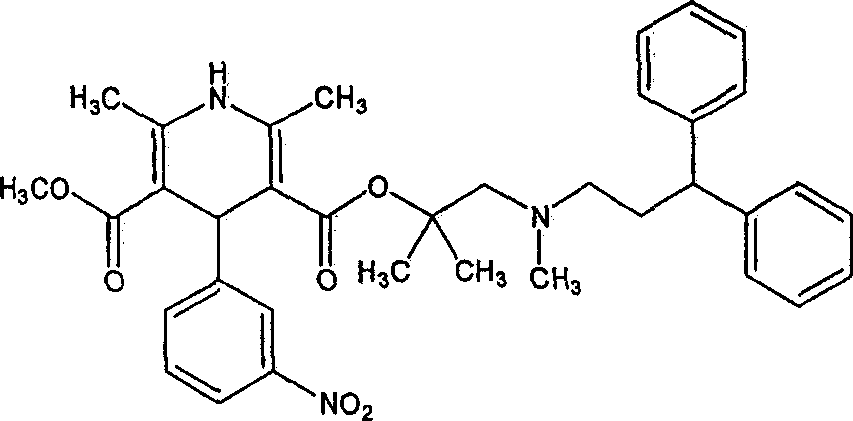
Pharmaceutical compositions comprising lercanidipine
A technology of lercanidipine and composition, which is applied in the field of controlled-release preparations and controlled-release pharmaceutical compositions, and can solve problems such as inconsistency in absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0277] Matrix capsules with intragranular hydrocolloids
[0278] substance
[0279] 20 g of lercanidipine were dissolved in a molten mixture of polyethylene glycol 6000 and poloxamer 188 (70:30) at 90°C. 318 g of the solid dispersion were sprayed onto a mixture of 150 g of lactose and 100 g of Metolose 90SH 100 cP in the fluid bed Strea-1. The granular product was screened through a 0.7mm sieve. The granular product was sieved through a 0.7 mm sieve and filled into hard gelatin capsules.
Embodiment 2
[0281] Matrix tablet with extragranular hydrocolloids
[0282] substance
[0283] The granulated product from Example 1 was mixed in a turbula mixer with 20% Metolose HS90 15000 cP for 3 minutes and then with 0.5% magnesium stearate for 5 minutes. The granules were directly compressed into 12 mm tablets (compound cups) in a Diaf TM20. The tablet has an average weight of 623 mg and a strength of 10 mg. Average tablet hardness: 51N.
Embodiment 3
[0285] Lercanidipine Capsules
[0286] substance
[0287] 2.5 g of lercanidipine were dissolved in 22.5 g of glyceryl monocaprylate at about 100°C. The clear solution was injected into 200mg capsules (size 1CS).
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com