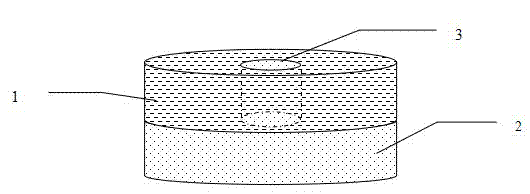
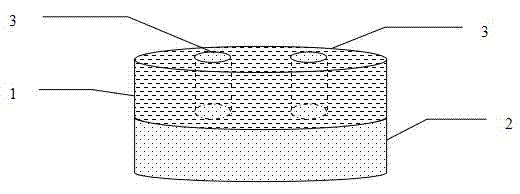
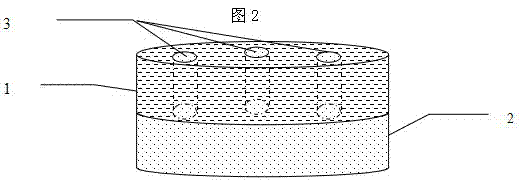
Hypnotic double-layer controlled release tablet and preparation method thereof
A controlled-release tablet, double-layer technology, applied in the direction of pharmaceutical formulations, medical preparations containing active ingredients, pill delivery, etc., can solve problems such as tablet splitting, achieve difficult separation, good physical stability, improve effectiveness and safety effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] The prescription of the double-layer controlled-release tablet of the present embodiment is as shown in Table 1. Its preparation method is as follows:
[0049] 1. Preparation of sustained-release granules:
[0050] ① Weigh the Zolpidem Tartrate that has passed the 60-mesh sieve and mix it with Hypromellose K100LV, K4M and lactose monohydrate, then add half of the prescription amount of silicon dioxide and magnesium stearate . Transfer the mixed material to the wet granulator;
[0051] ② Prepare an ethanol solution with a concentration of 70% to make soft materials from the mixture in step 1;
[0052] ③Sizing the prepared soft material with a 24-mesh sieve;
[0053] ④ dry and sieve the particles;
[0054] ⑤ Obtaining sustained-release granules for forming a sustained-release layer.
[0055] 2. Preparation of immediate-release granules:
[0056] ① Mix the prescribed amount of zolpidem tartrate, lactose, microcrystalline cellulose, and povidone through a 60-mesh sie...
Embodiment 2
[0069] The prescription of the double-layer controlled-release tablet of the present embodiment is as shown in Table 1. Its preparation method is as follows:
[0070] 1. Preparation of sustained-release granules:
[0071] ①Weigh the prescription amount of zolpidem tartrate that has passed through a 60-mesh sieve, mix well with hypromellose K100LV, K4M, and lactose, and then add half of the prescription amount of silicon dioxide and magnesium stearate. Transfer the mixed material to the wet granulator;
[0072] ②Prepare an ethanol solution with a concentration of 75% to make soft materials from the mixture in step 1;
[0073] ③Sizing the prepared soft material with a 24-mesh sieve;
[0074] ④ dry and sieve the particles;
[0075] ⑤ Obtaining sustained-release granules for forming a sustained-release layer.
[0076] 2. Preparation of immediate-release granules:
[0077] ① Mix the prescribed amount of zolpidem tartrate, lactose, microcrystalline cellulose, hydroxypropyl cel...
Embodiment 3
[0089] The prescription of the double-layer controlled-release tablet of the present embodiment is as shown in Table 1. Its preparation method is as follows:
[0090] 1. Preparation of sustained-release granules:
[0091] ①Weigh the prescribed amount of zopiclone that has passed through a 60-mesh sieve, mix well with hypromellose K100LV, K4M, and lactose, and then add half of the prescribed amount of silicon dioxide and magnesium stearate. Transfer the mixed material to the wet granulator;
[0092] ② Prepare an ethanol solution with a concentration of 80% to make a soft material from the mixture in step 1;
[0093] ③Sizing the prepared soft material with a 24-mesh sieve;
[0094] ④ dry and sieve the particles;
[0095] ⑤ Obtaining sustained-release granules for forming a sustained-release layer.
[0096] 2. Preparation of immediate-release granules:
[0097] ①Mix the prescription amount of zopiclone, lactose, microcrystalline cellulose, hydroxypropyl cellulose-HF, and so...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com