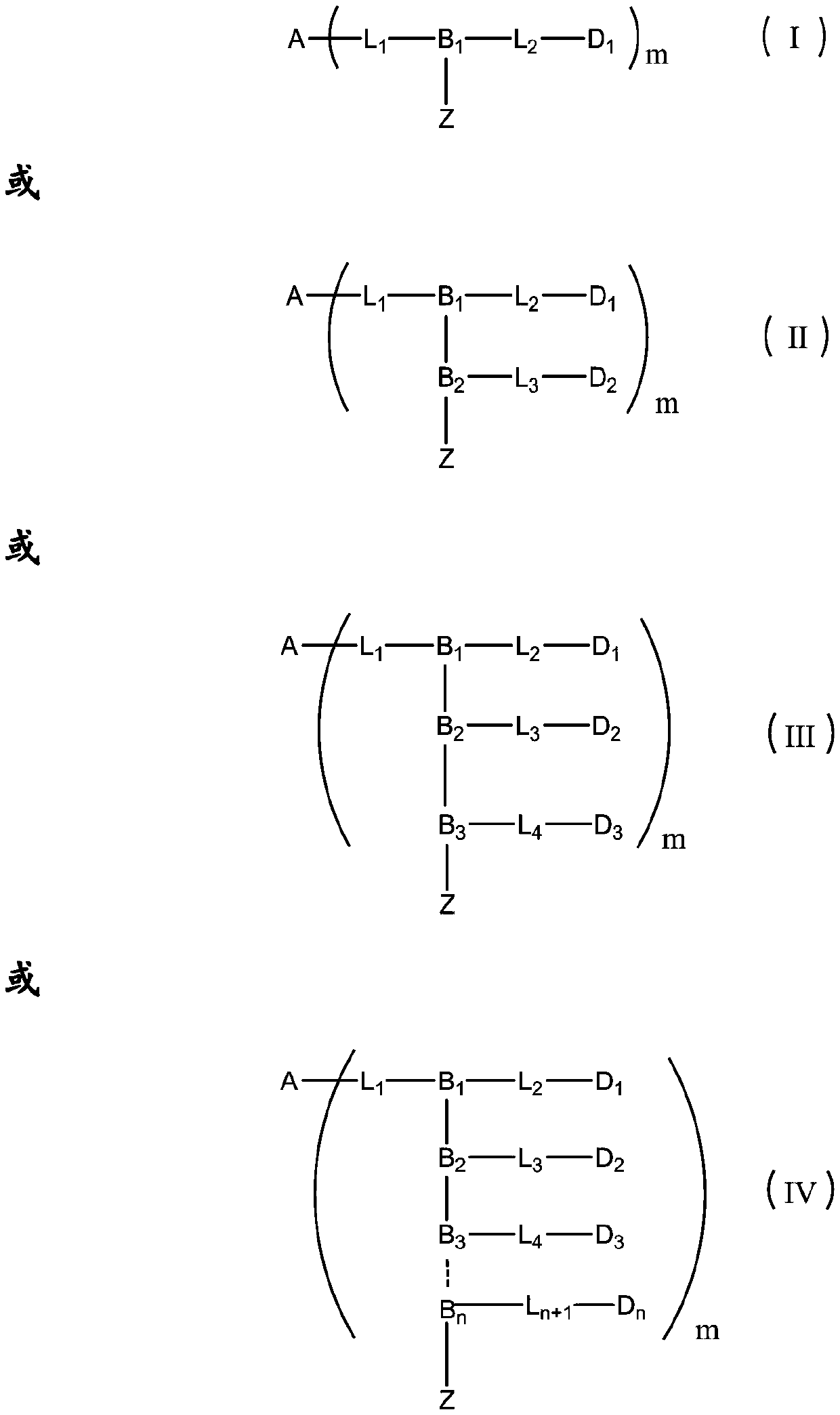
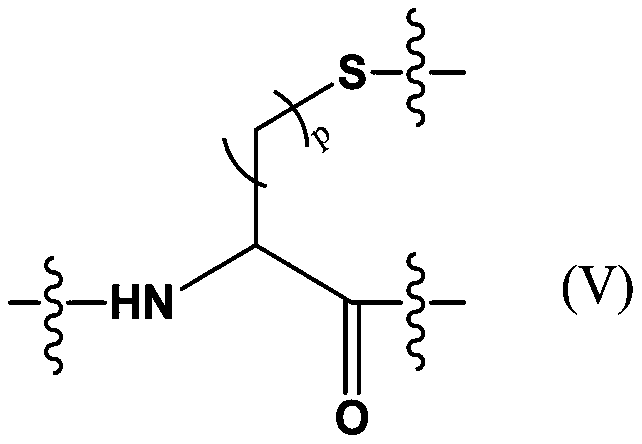
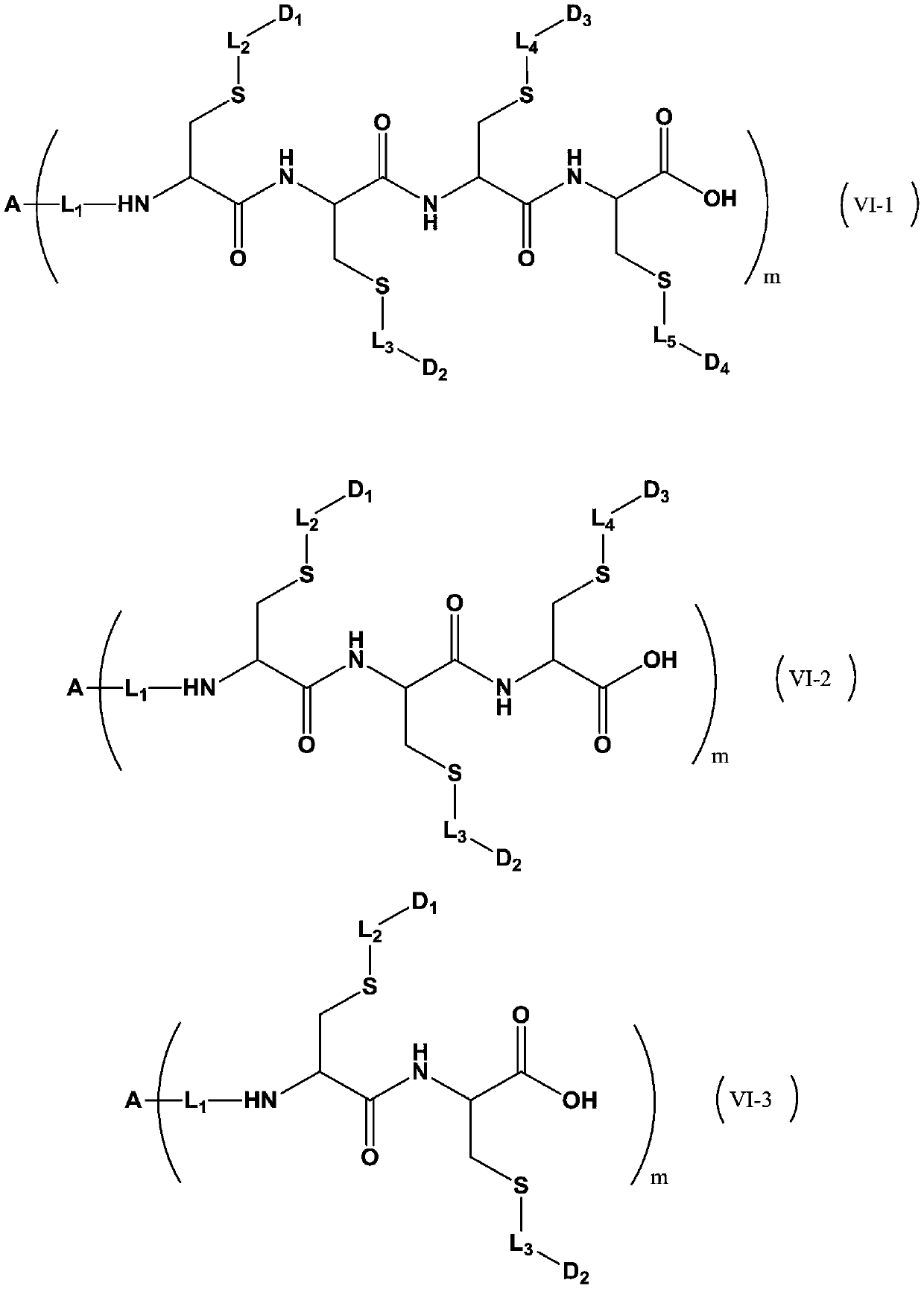
Antibody drug conjugate and application thereof
A technology of antibody drugs and conjugates, applied in the field of biomedicine, can solve problems such as failure, low quantity of small molecule drugs, and low drug efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment
[0172] Below in conjunction with specific embodiment, further illustrate the present invention. It should be understood that these examples are only used to illustrate the present invention and are not intended to limit the scope of the present invention. The experimental methods in the following examples that do not indicate specific conditions are usually carried out according to conventional conditions or according to the conditions suggested by the manufacturer, and the reagents that do not indicate specific sources are conventional reagents purchased in the market. All percentages, ratios, ratios, or parts are by weight unless otherwise indicated.
[0173] The unit of weight volume percentage in the present invention is well known to those skilled in the art, for example, it refers to the weight of solute in 100 ml of solution.
[0174] Unless otherwise defined, all professional and scientific terms used herein have the same meanings as those familiar to those skilled in...
Embodiment 1
[0175] The preparation of embodiment 1 compound 1
[0176]
[0177] Dissolve 145.4mg maleimide caproic acid in 10mL DMF, add 244.7mg HATU and 226uLDIPEA, and stir at room temperature. 201.9mgMMAF was dissolved in 5mL DMF, slowly added dropwise to the above reaction system, and stirred at room temperature for 16h. The solvent was distilled off under reduced pressure and purified by preparative liquid phase to obtain 90.3 mg of the product (maleimidecaproyl-MMAF, Mc-MMAF) with a yield of 36%. LC-MS: (M+H) + 924.8, (M-H) - 923.2.
[0178] Dissolve 36.1mg of maleimidocaproyl-MMAF in 1.5mL DMF, add 5.6mg of Cys-Cys and 2.1uLDIPEA, stir at room temperature for 3h, then add 1.1mg of Cys-Cys, and stir at room temperature for 2h. Add 17.7 mg of 6-(maleimido) hexanoic acid succinimidyl ester and 25 uL DIPEA, and stir at room temperature for 15 h. The solvent was distilled off under reduced pressure and purified by preparative liquid phase method to obtain 120.0 mg of compound wi...
Embodiment 2
[0179] The preparation of embodiment 2 compound 2
[0180]
[0181] According to the preparation method of Example 1, maleimide caproyl-MMAF was prepared. Dissolve 40.1mg maleimide caproyl-MMAF in 1.5mL DMF, add 4.4mg Cys-Cys-Cys and 2.1uLDIPEA, stir at room temperature for 2h, then add 1.8mgCys-Cys-Cys, react at room temperature for 2h, then add 0.9mg Cys-Cys-Cys, react at room temperature for 2h. Add 13.5 mg 6-(maleimido) hexanoic acid succinimide ester and 21 uLDIPEA, and stir at room temperature for 15 h. The solvent was distilled off under reduced pressure and purified by preparative liquid phase method to obtain 223.1 mg of compound with a yield of 49%. LC-MS: (M+3H) 3+ 1648.4, (M-3H) 3- 1646.3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com