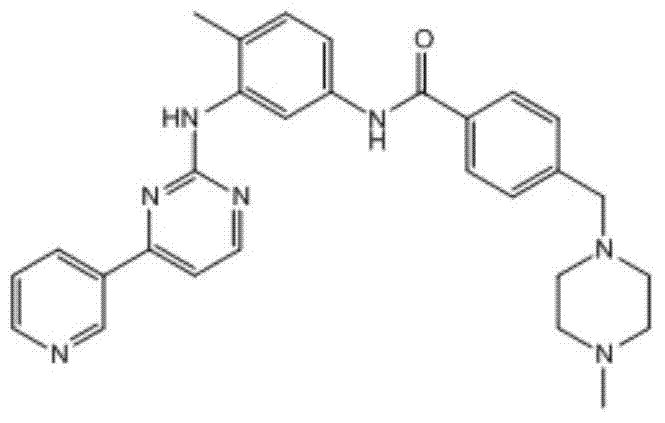
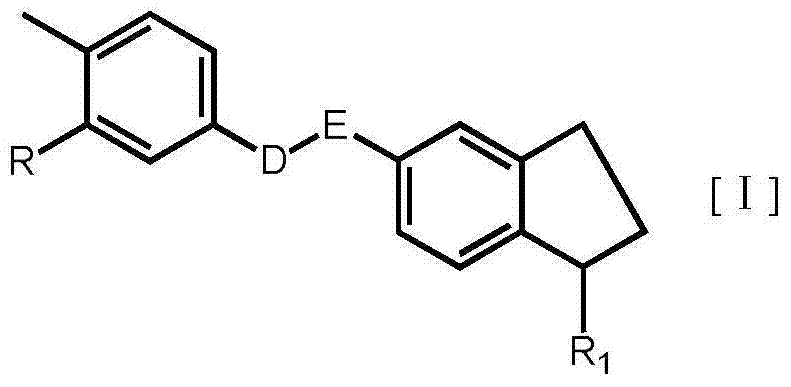
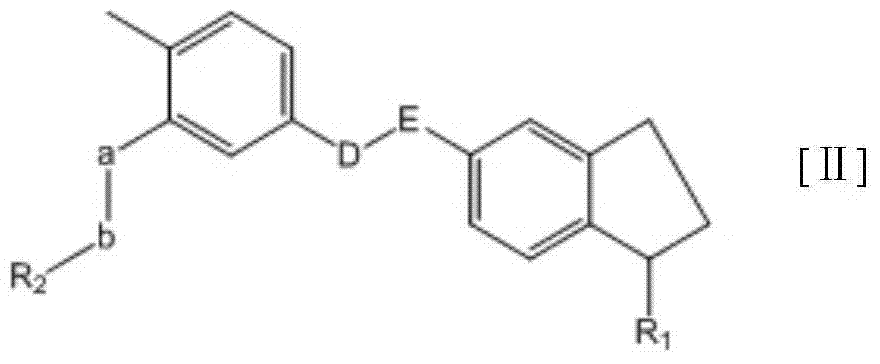
Substituted-indanyl amide-type compounds and pharmaceutically acceptable salt and preparation method and application thereof
A technology of dihydroindane amide and compound, applied in the field of medicine, can solve problems such as death, adverse reactions of patients, steric hindrance, etc., and achieve the effects of broad anti-cancer spectrum, excellent anti-tumor activity and safety, and wide therapeutic window.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Example 1 3-(imidazo[1,2-b]pyridazin-3-ethynyl)-4-methyl-[N-5-(4-methylpiperazinyl)-tetrahydroindane]benzyl Synthesis of Amide (Compound 1)
[0051]
[0052] 1. Synthesis of 5-fluorenylmethoxycarbonyl amido-indanone
[0053]
[0054] Under the protection of nitrogen, add stirring bar, 5-aminoindanone (14.7 grams, 0.1 moles), 200 milliliters of dichloromethane solution, pyridine (23.7 grams, 0.3 moles) in 500 milliliters of eggplant-shaped bottles, stir while ice-water bath Fluorenemoxycarbonyl chloride (25.8 g, 0.1 mol) was slowly added dropwise in portions. After the dropwise addition, the temperature was raised to room temperature and stirring was continued for 2 hours. The reaction solution was quenched by adding 200 ml of water, the dichloromethane phase was separated and washed with 100 ml of brine, the organic phase was dried over anhydrous sodium sulfate, concentrated in vacuo, and separated by column chromatography to obtain 33.8 g of the product with a ...
Embodiment 2
[0077] Example 2 3-(imidazo[1,2-a]pyrimidine-3-ethynyl)-4-methyl-[N-5-(1-imidazolyl)-indane]benzamide (compound 2 )Synthesis
[0078]
[0079] 1. Synthesis of 1-(1-imidazolyl)-5-fluorenylmethoxycarbonamidotetrahydroindene
[0080]
[0081] Add 1-chloro-6-fluorenylmethoxycarbonamidotetrahydroindane (3.11 g, 8 mmol), potassium carbonate (3.31 g, 24 mmol), 40 ml of tetrahydrofuran solvent in a 100 ml eggplant-shaped bottle, N, 10 ml of N-dimethylformamide and N-methylpiperazine (1.00 g, 10 mmol) were stirred at 40° C. for 4 hours to stop the reaction. After the reaction solution was concentrated under reduced pressure, 50 ml of ethyl acetate was added to dissolve it. And washed 3 times with 50 ml of saturated saline. The organic phase was separated, dried over anhydrous sodium sulfate, concentrated under reduced pressure, and separated by column chromatography to obtain 3.06 g of the product with a yield of 91%. MS: m / z 422 ([M+H] + ).
[0082]2. Synthesis of 1-(1-imid...
Embodiment 3
[0098] Example 3 3-(imidazo[1,2-a]pyrimidin-3-ethynyl)-4-methyl-[N-5-(4-tert-butoxyyl)-tetrahydroindene]benzamide ( Compound 3) Synthesis
[0099]
[0100] 1. Synthesis of 1-(4-tert-butoxyacyl)-5-fluorenylmethoxycarbonamidotetrahydroindene
[0101]
[0102] Add 1-chloro-6-fluorenylmethoxycarbonamidotetrahydroindane (3.11 g, 8 mmol), potassium carbonate (3.31 g, 24 mmol), 40 ml of tetrahydrofuran solvent in a 100 ml eggplant-shaped bottle, N, 10 ml of N-dimethylformamide and N-methylpiperazine (1.00 g, 10 mmol) were stirred at 40° C. for 4 hours to stop the reaction. After the reaction solution was concentrated under reduced pressure, 50 ml of ethyl acetate was added to dissolve it. And washed 3 times with 50 ml of saturated saline. The organic phase was separated, dried over anhydrous sodium sulfate, concentrated under reduced pressure, and separated by column chromatography to obtain 3.62 g of the product with a yield of 84%. MS: m / z 540 ([M+H] + ).
[0103] 2. Syn...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com