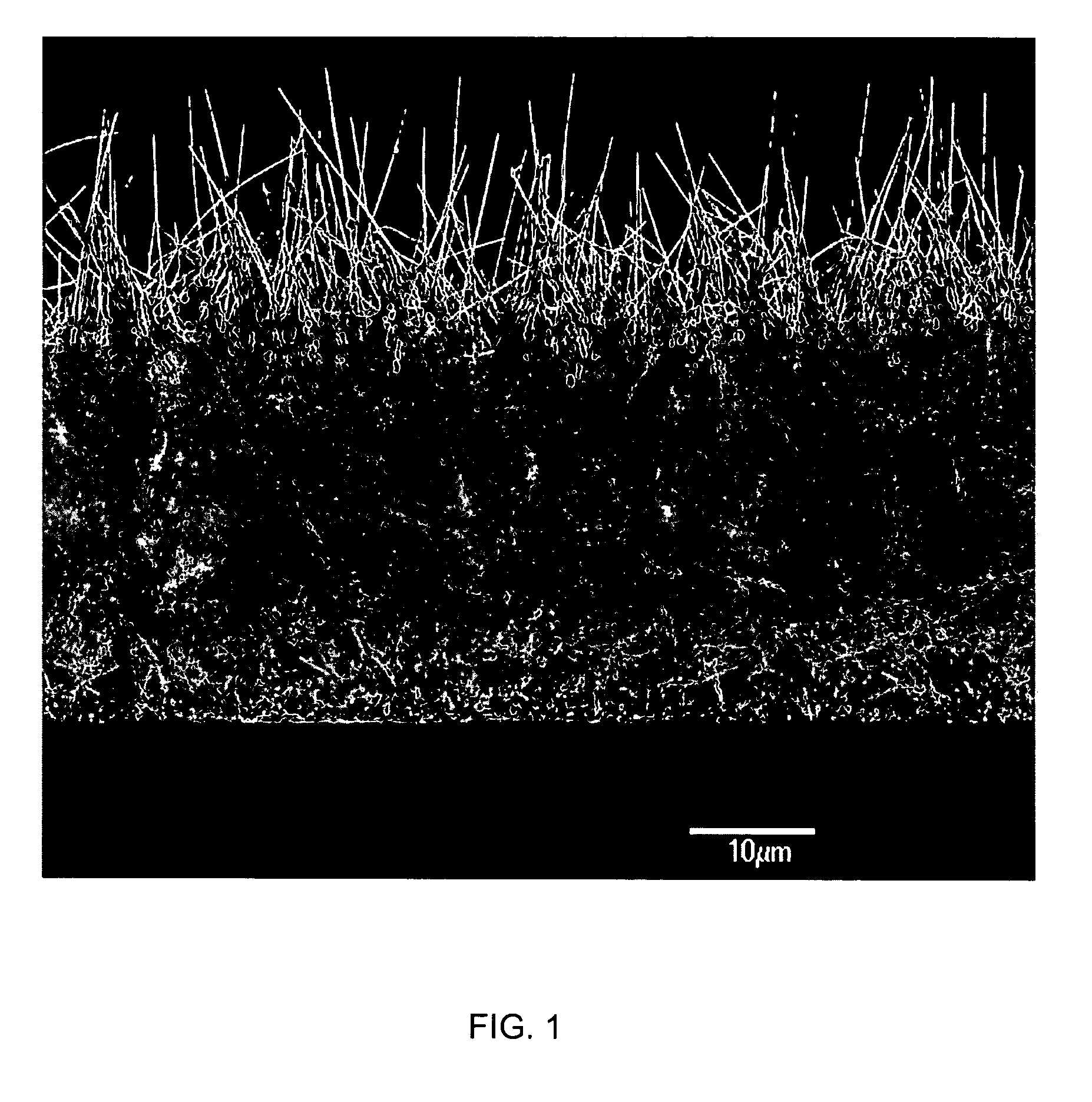
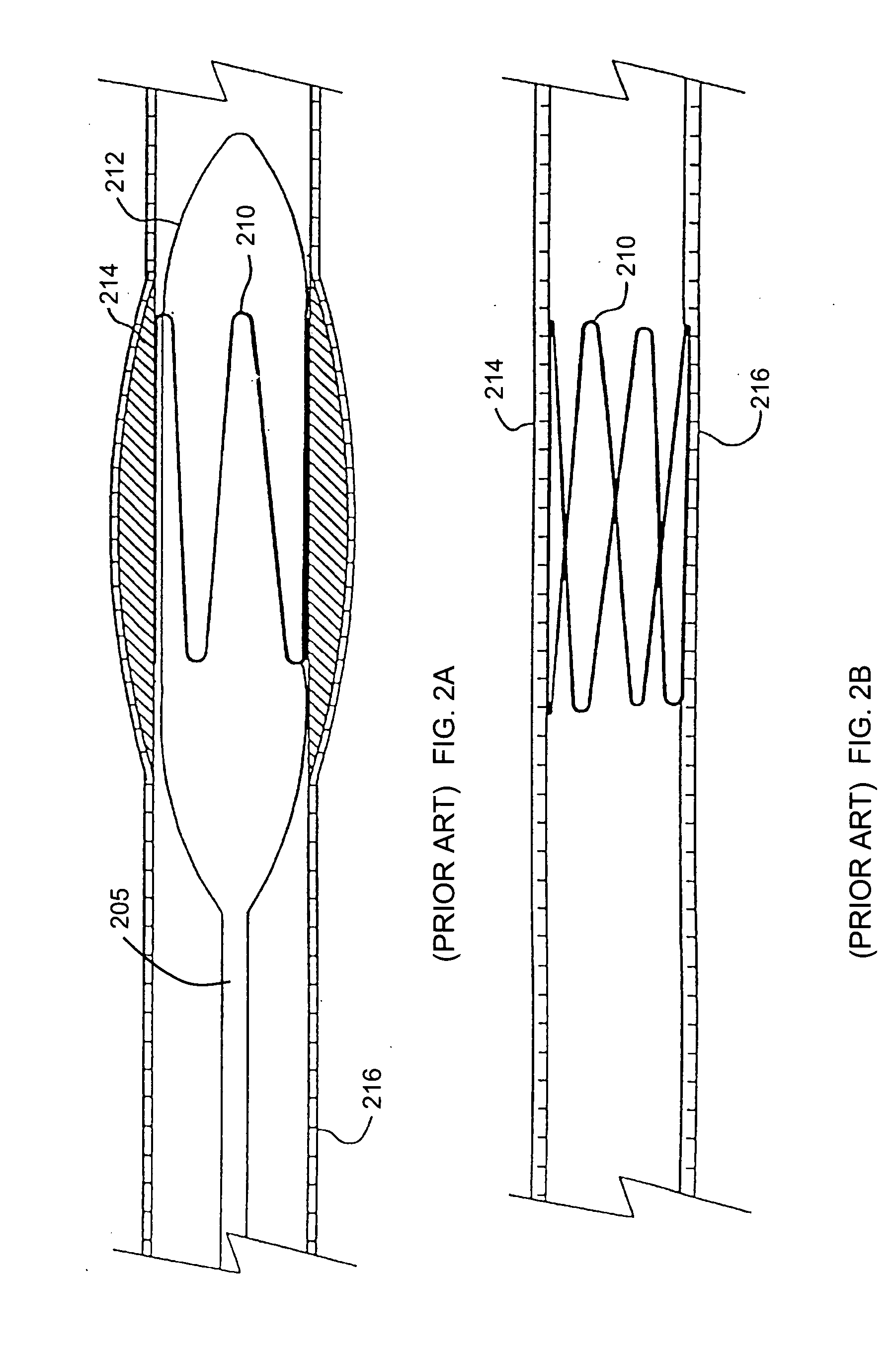
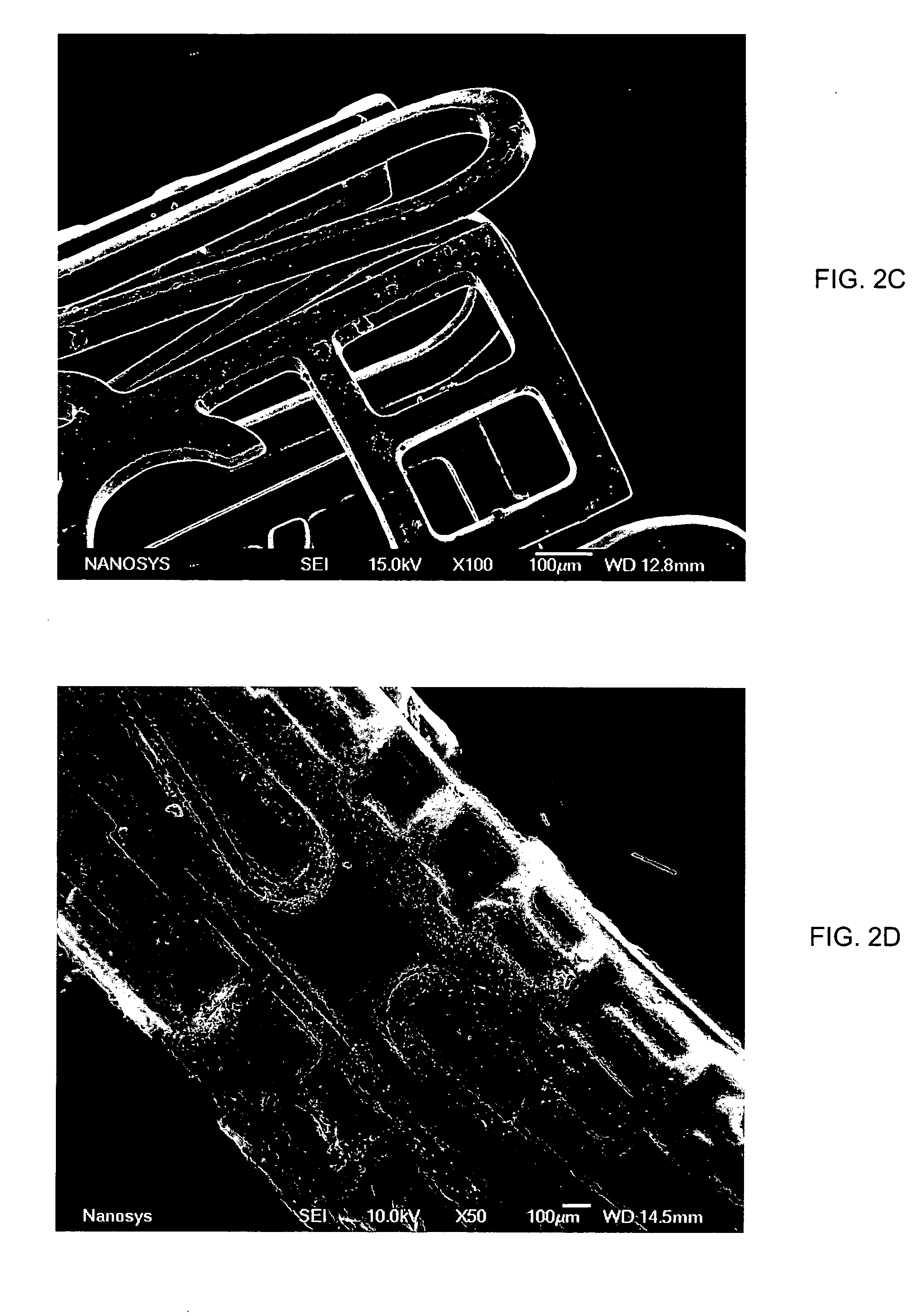
Medical device applications of nanostructured surfaces
a technology of nanofibers and surfaces, applied in the field of nanotechnology, can solve problems such as endangering and susceptible to damage, and achieve the effects of reducing bio-fouling, increasing fluid flow, and improving surface appearan
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0262] The following non-limiting Example presents data from a study conducted at Boston University that illustrates how the use of nanofiber (e.g., nanowire) surfaces as compared to control (reference) surfaces (e.g., quartz) for bone biotemplating applications helps in faster cell differentiation which can be expected to result in faster bone in-growth.
Osteoblast Culture
[0263] Human fetal osteoblasts, designated hFOB 1.19 (American Type Culture Collection (ATCC), Manassas, Va.), were used for cell adhesion studies. This cell line was obtained from a spontaneous miscarriage and transfected with a temperature-sensitive mutant gene of SV40 large T antigen. The cells were programmed to proliferate at 34° C. and differentiate only when the temperature is raised to 39° C. Cells with passage 10 were used in all experiments. The medium used for growing osteoblasts consisted of 1:1 ratio of DMEM:F12 (Invitrogen Corp.) with 10% fetal bovine serum (Sigma-Aldrich) and 0.3 mg / mL of G418 sul...
example 2
[0272] The following non-limiting Example presents data from a Purdue University study that illustrates how the use of nanofiber (e.g., nanowire) surfaces as compared to current orthopedic implant materials leads to increased select osteoblast adhesion in a competitive cell adhesive environment. Various cells important for orthopedic applications were allowed to interact with: current implant materials (i.e., commercially pure titanium (Ti), Ti6Al4V, and CoCrMo), current implant materials with a bioactive hydroxyapatite (HA) coating (i.e., Ti coated with HA and Ti6Al4V coated with HA), HA used not as a coating but in bulk, and nanowire surfaces. Cells that were allowed to interact with the materials simultaneously to simulate in vivo conditions were: osteoblasts (bone-forming cells), fibroblasts (fibrous, not hard, tissue forming cells), endothelial cells, and smooth muscle cells. Fibroblasts, endothelial cells, and smooth muscle cells are considered competitive cells to osteoblasts...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com