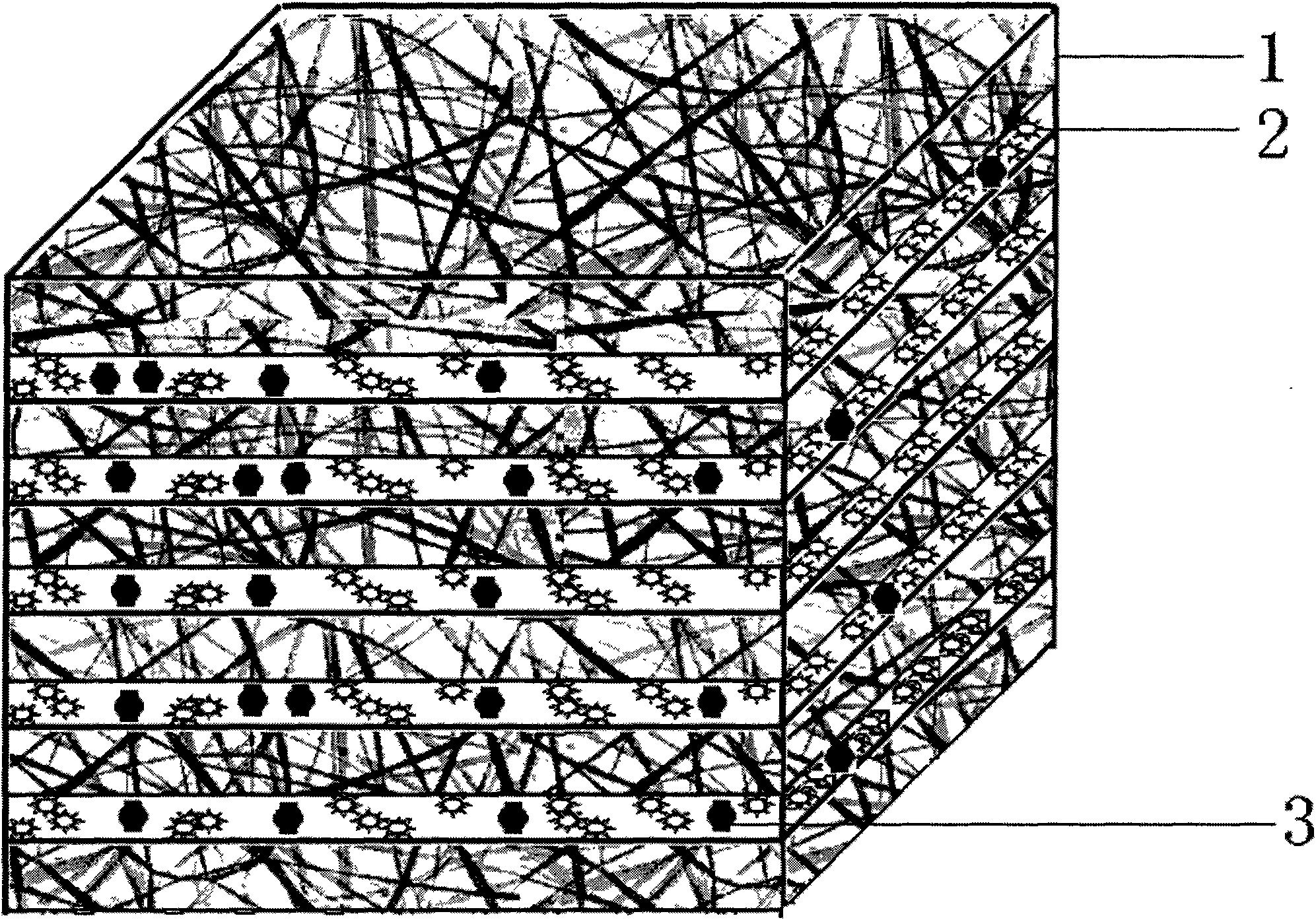
Artificial articular cartilage based on autologous cells and preparation method thereof
A technology of artificial joints and autologous cells, applied in the field of biological doctors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] (1) Prepare electrospinning solution, hydrosol solution containing cytokines and cross-linking agent solution;
[0070] The electrospinning solution is preferably a dichloromethane solution of poly-DL-lactic acid and polycaprolactone with a number average molecular weight of 26000; the weight percentages of the poly-DL-lactic acid and polycaprolactone are 50% and 50% respectively , the total mass percent concentration of the solution is 7%.
[0071] The cross-linking agent solution is 0.1M calcium chloride solution.
[0072] The hydrosol solution containing cytokines adopts alginate solution, and the mass percent concentrations of cytokines TGF-β1, IGF-1, and BMP-2 in the cytokine alginate solution are each 100 ppm.
[0073] Put the prepared 0.1M calcium chloride solution into a cell culture dish with a diameter of 150 mm, and place it on the flat receiver shared by the electrospinning device and the printer. The Hewlett-Packard 550C inkjet printer was refitted accord...
Embodiment 2
[0081] Implementation steps are the same as in Example 1.
[0082] The preferred number-average molecular weight of the electrospinning solution is a dichloromethane solution of poly-DL-lactic acid and polycaprolactone of 26000; the weight percentages of the poly-DL-lactic acid and polycaprolactone are respectively 50% and 50%. 50%, the total mass percent concentration of the solution is 5%.
[0083] The cross-linking agent solution is 100IU / ml thrombin solution;
[0084] The hydrosol solution containing cytokines adopts cytokine fibrinogen solution, and the concentration of BMP-2 is 10 mg / ml.
[0085] The specific operation is to put the configured thrombin solution into a cell culture dish with a diameter of 90 mm, and place it on the flat receiver shared by the electrospinning device and the printer. The Hewlett-Packard 550C inkjet printer was refitted according to the existing patent reports, for example, referring to the method disclosed in US Patent No. 7,051,654, and ...
Embodiment 3
[0089] The preferred number-average molecular weight of the electrospinning solution is a dichloromethane solution of poly-DL-lactic acid and polycaprolactone of 26000; the weight percentages of the poly-DL-lactic acid and polycaprolactone are respectively 50% and 50%. 50%, the total mass percent concentration of the solution is 10%.
[0090] The crosslinking agent solution is selected from 100 mg / ml water-soluble carbodiimide solution;
[0091] The hydrosol solution containing cytokines adopts collagen and polyanion solution of cytokines, wherein the collagen concentration is 1%, polyanion adopts polyglutamic acid with a concentration of 50 mg / ml, and the cytokines include cell directional migration factor SDF-1, The total mass percent concentration of epidermal growth factor, transforming growth factor beta, BMP-2 and dexamethasone is 0.5%.
[0092] The specific operation is to put the prepared carbodiimide solution into a cell culture dish with a diameter of 150mm, and put...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com