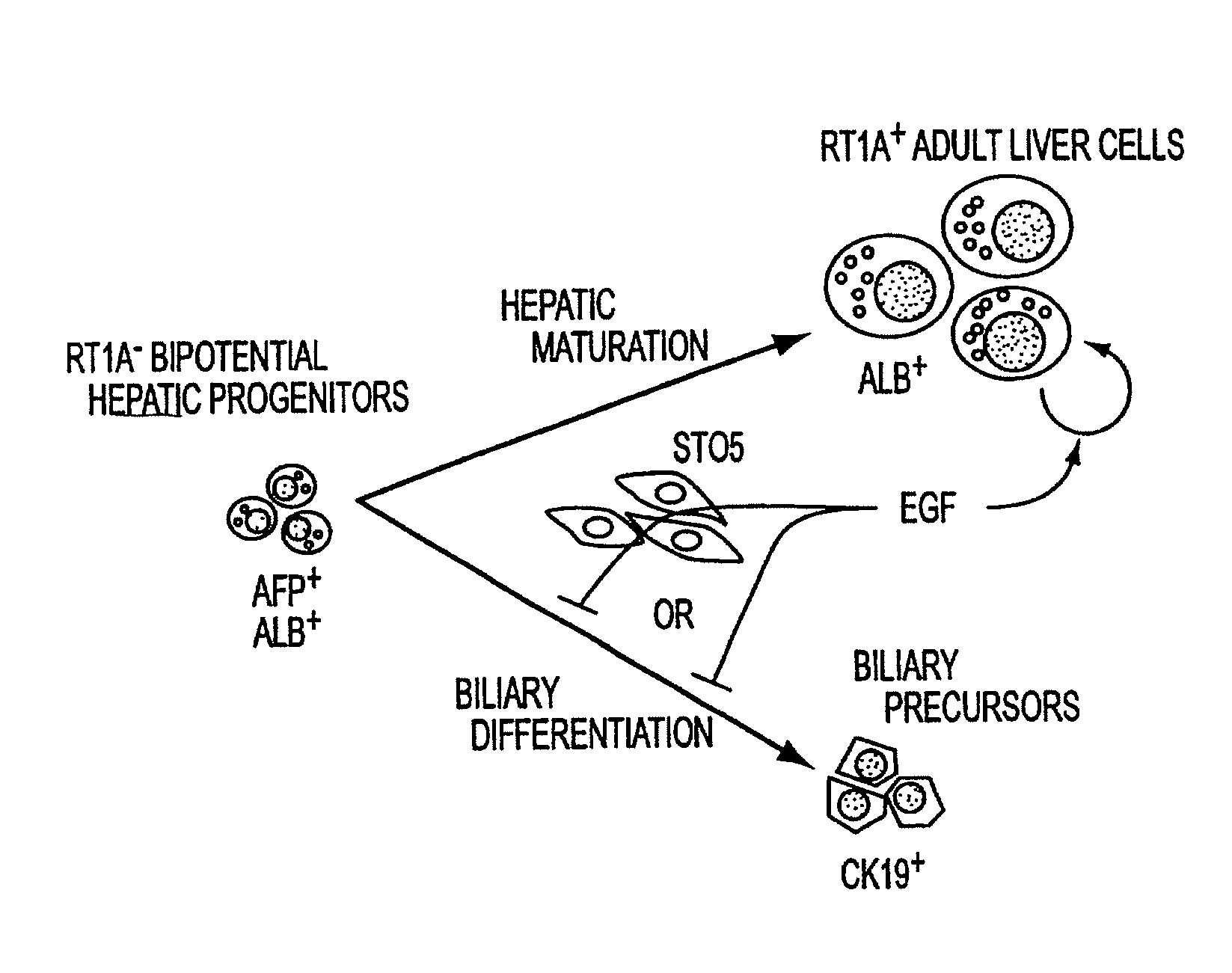
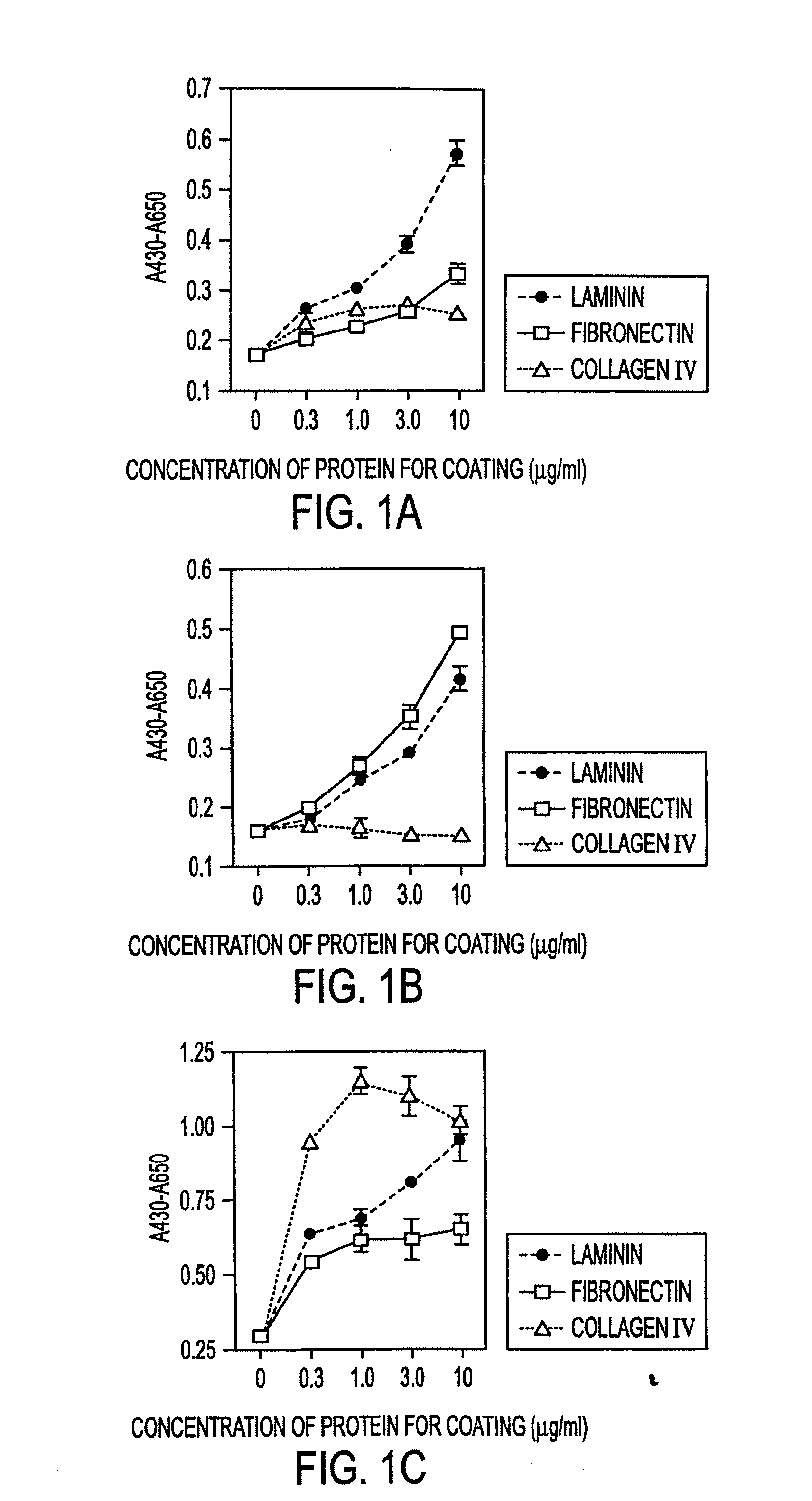
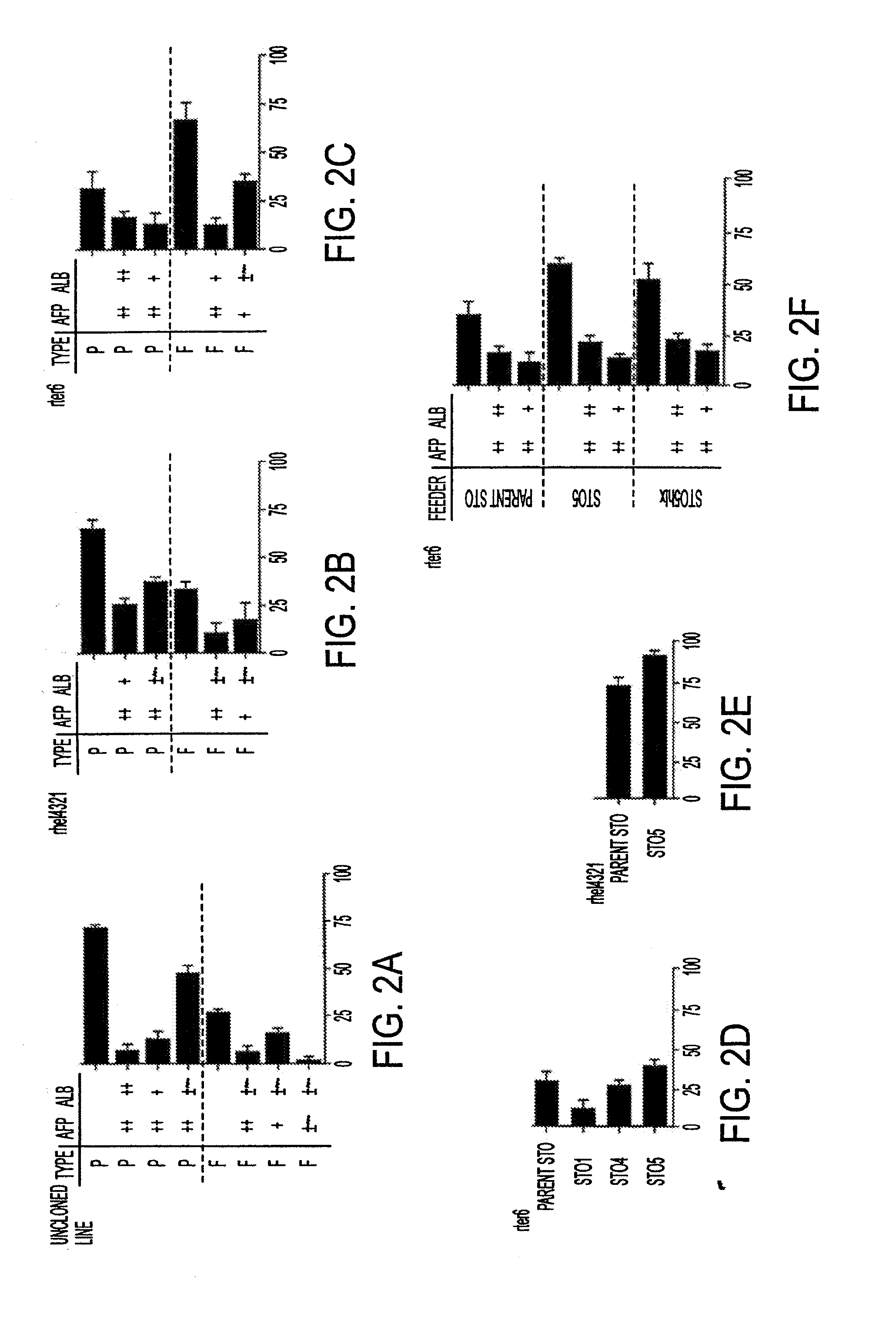
Methods of isolating bipotent hepatic progenitor cells
a hepatic progenitor cell and hepatic technology, applied in the field of new cell surface markers, can solve the problems of inability to test for bipotent cell populations, inability to detect biliary epithelial cells, and inability to detect hematopoietic cells, and achieve the effect of higher side scatter
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0036] Glossary
[0037] Classical MHC class I antigen. The group of major histocompatability antigens commonly found mostly on all nucleated cells although they are most highly expressed on hematopoietic cells. The antigen is also known as MDHC class Ia. The nomenclature of the classical NHC antigens is a function of species, for example in humans the MHC antigens are termed HLA. Table 3 provides nomenclature of classical MHC antigens in several species.
[0038] Non classical MHC class I antigen. The group of major histocompatability antigens, also known as MHC class lb. that can vary even within a species. The nomenclature of the nonclassical MHC antigens varies by species, see, e.g., Table 4.
[0039] ICAM Intercellular adhesion molecule-1 (CD54) is a membrane glycoprotein and a member of the immunoglobulin superfamily. The ligands for ICAM-1 are the β2-integrin, LFA-1 (CD11a / CD 18) and Mac-i (CD11b / CD18). This molecule is also important for leukocyte attachment to endothelium. In add...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| body weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com