Beta-cyclodextrin-modified polyamide-dendrimer and preparation method of gold nanoparticle compound of beta-cyclodextrin-modified polyamide-dendrimer
A technology of amine dendrimers and dendrimers, which is applied in the field of preparation of polyamide-dendrimer and its nano-gold particle complex, to avoid aggregation, good gene transfection effect, and improve biocompatibility Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
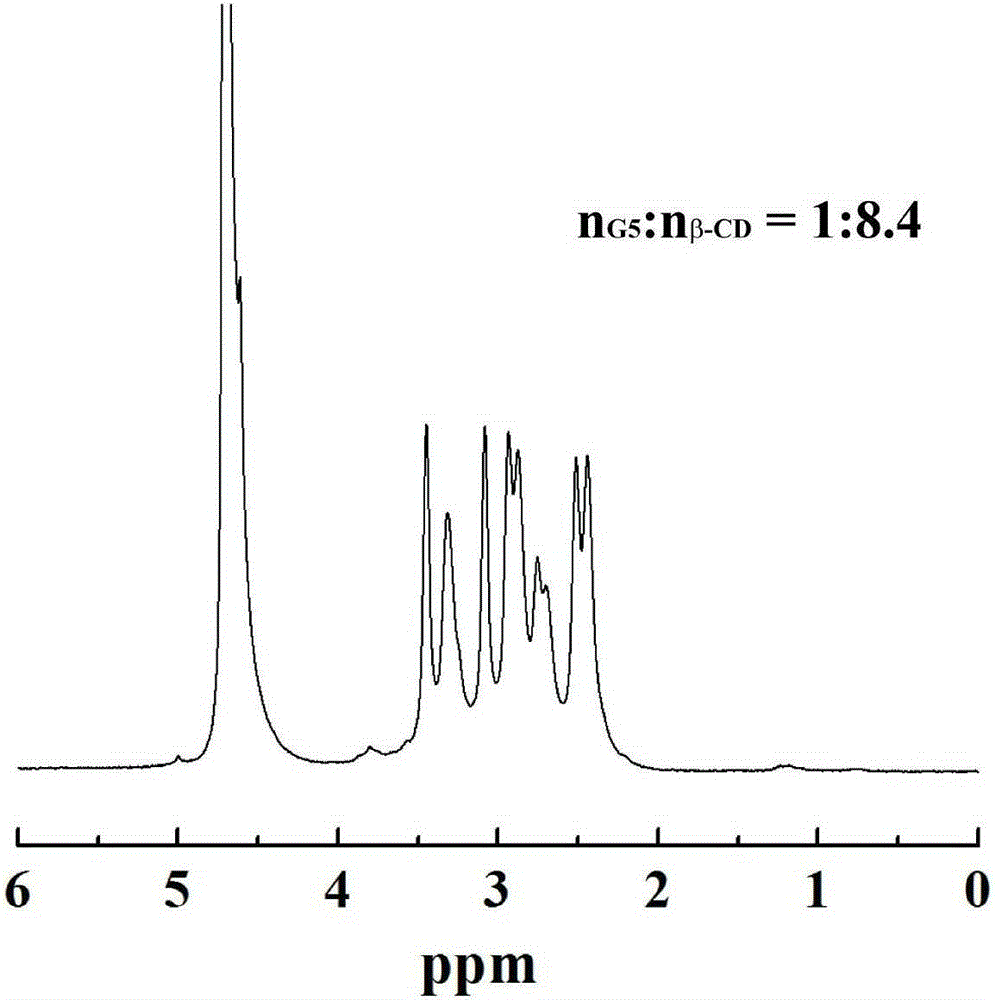
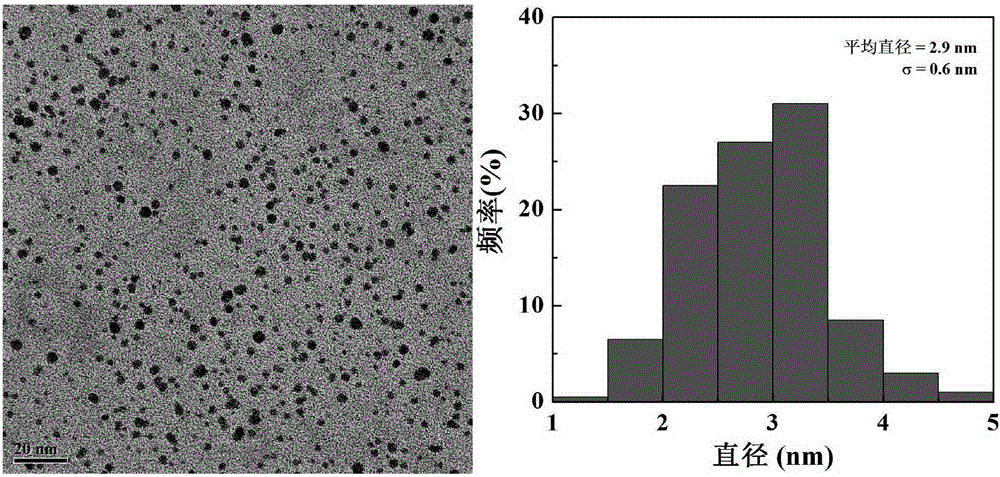
[0036] Weigh 16.46mg β-CD and 23.51mg CDI, dissolve them in 5mL DMSO respectively, stir and react for 6h. Weigh the fifth generation polyamidoamine dendrimer (G5.NH 2 ) 15.06 mg, dissolved in 5 mL DMSO. Add the activated β-CD solution to G5.NH dropwise 2 solution, magnetically stirred at room temperature, and reacted for 3d to obtain G5.NH 2 -β-CD. G5.NH 2 - β-CD was transferred to a dialysis bag with a molecular weight cut-off of 14000, dialyzed in distilled water for three days (2L×3), and then freeze-dried, and finally the obtained G5.NH 2 - β-CD was dissolved in 5 mL of water, and 322.7 μL of HAuCl was added dropwise 4 Aqueous solution (30mg / mL) was mixed and stirred for 30min, then 445μL of 10mg / mL NaBH was added rapidly 4 solution, reacted for 3h, and obtained {(Au 0 ) 25 -G5.NH 2 -β-CD} solution. After the reaction, the reaction product {(Au 0 ) 25 -G5.NH 2 -β-CD} was transferred to a dialysis bag with a molecular weight cut-off of 14000, and dialyzed in di...
Embodiment 2
[0039] G5.NH 2 , the {(Au 0 ) 25 -G5.NH 2}, {(Au 0 ) 25 -G5.NH 2 -β-CD} was complexed with pDNA and subjected to gel retardation experiments. Prepare 8 wells of agarose gel (1.0% w / v) containing ethidium bromide (0.1 μg / mL), and place at room temperature until the agarose gel solidifies. Taking 1 μg of pDNA as an example, prepare vector / pDNA complexes according to different N / P (0.25, 0.5, 1, 2, 3, 4, 5), and use DNAmarker as a positive control. Then the corresponding vector / pDNA complexes were respectively added to the wells of the agarose gel with a voltage of 80V and a time of 30min. The migration of DNA in the gel was analyzed using a gel imager. The result is as Figure 4 shown. The results showed that {(Au 0 ) 25 -G5.NH 2} and {(Au 0 ) 25 -G5.NH 2 -β-CD} can well complex with DNA at a lower N / P (N / P=1) and block DNA. in, Figure 4 1 is the DNAmarker band, which is the positive control group, and the remaining 2-8 are vector / pDNA complexes prepared with ...
Embodiment 3
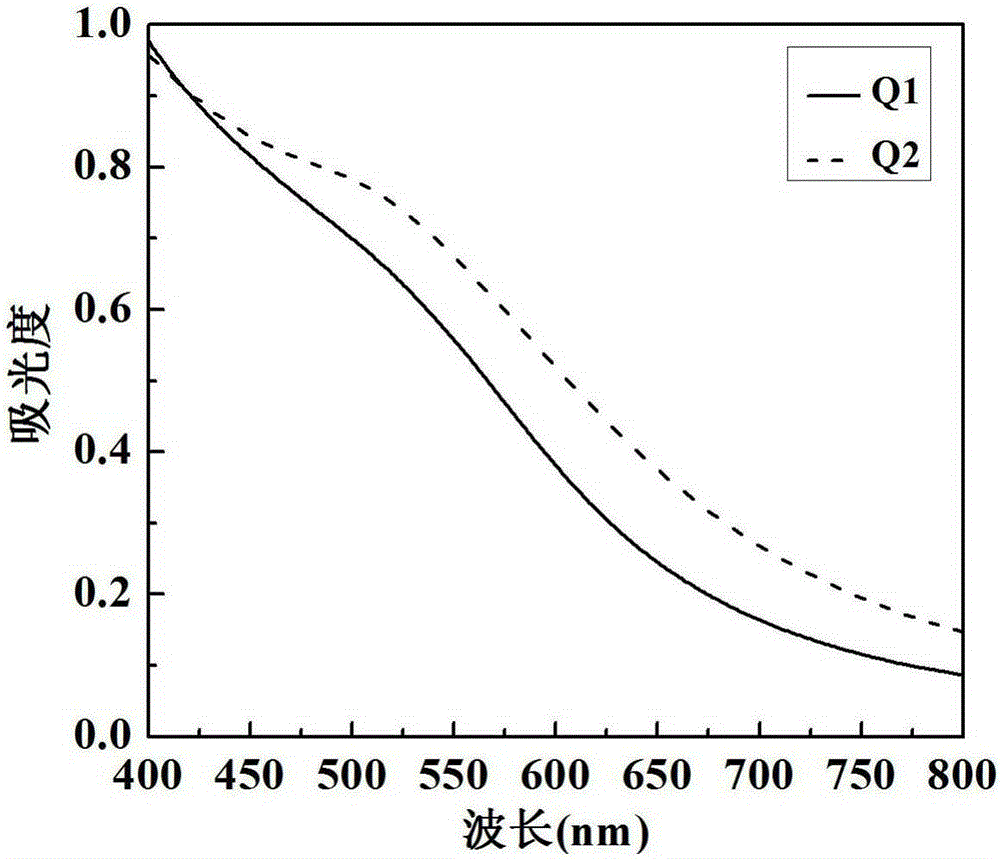
[0041] G5.NH 2 , the {(Au 0 ) 25 -G5.NH 2}, {(Au 0 ) 25 -G5.NH 2 -β-CD} under different N / P ratio conditions (1:1, 2.5:1, 5:1, 10:1) and 5 μg pDNA to form vector / pDNA complexes through electrostatic interaction, so that the final volume was fixed at 100 μL , incubate at room temperature for 20 min, and then add 1 mL of deionized water. Characterize its hydrodynamic particle size and surface potential by Malvern laser particle size analyzer (Malvern, MK, 633nm laser), the result is as follows Figure 5 shown. The results show that with the increase of N / P, the sizes and potentials of all the composites first decrease and then slightly increase; under the same N / P, the material potential of the coated gold nanoparticles is higher than that of the uncoated gold nanoparticles. However, the hydrodynamic particle size and surface of all functionalized polyamidoamine dendrimers and their nanocomposites / pDNA The potentials are all within the appropriate transfection range, wh...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com