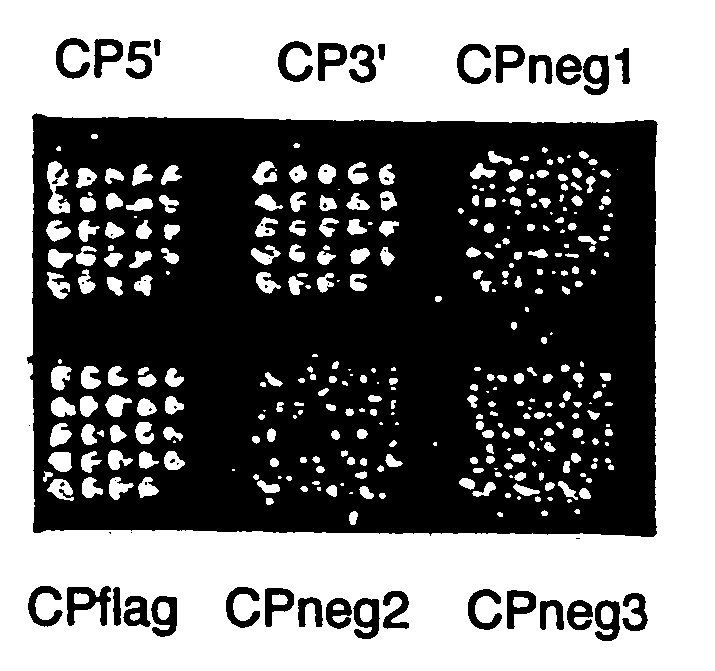
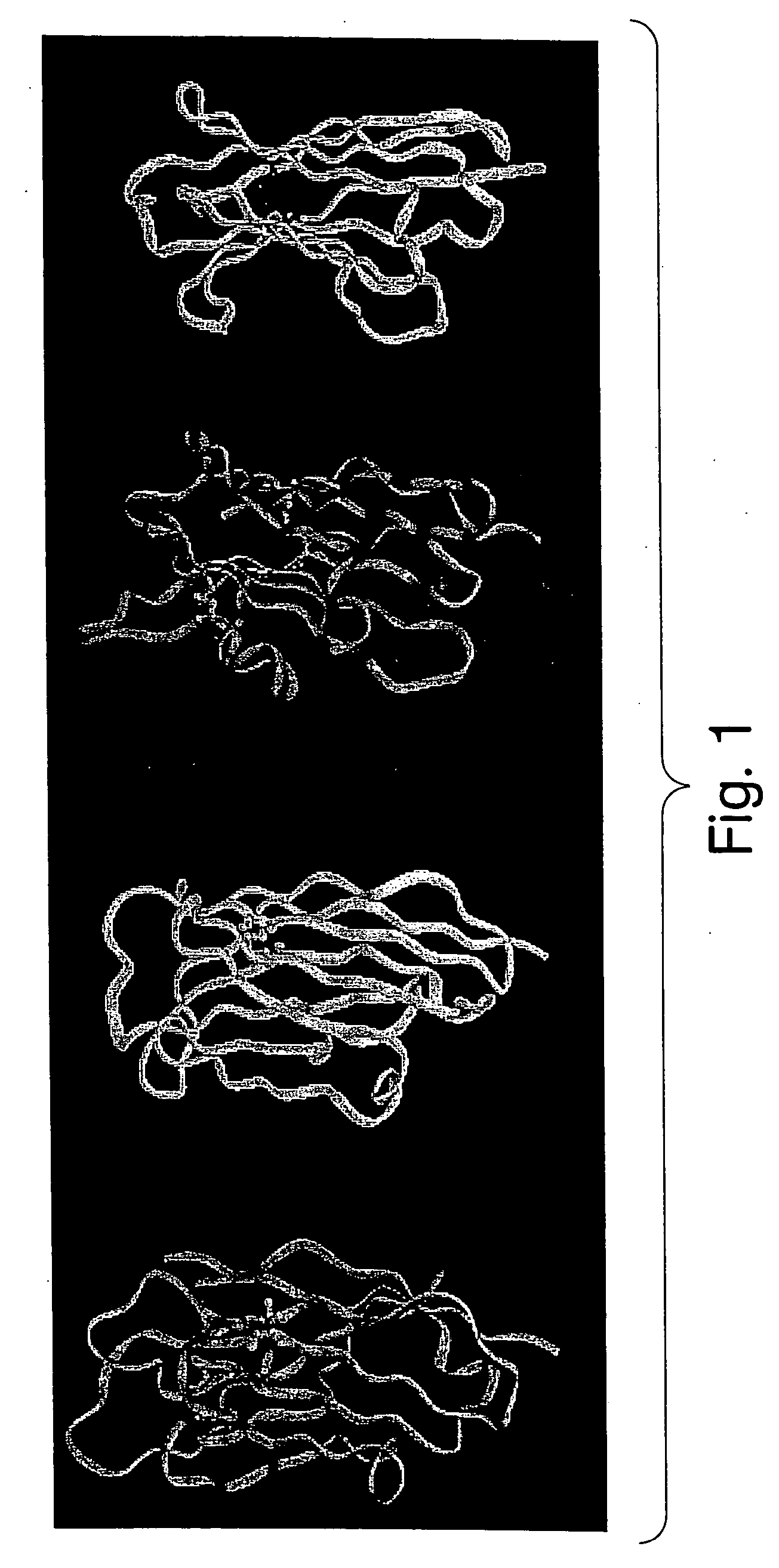
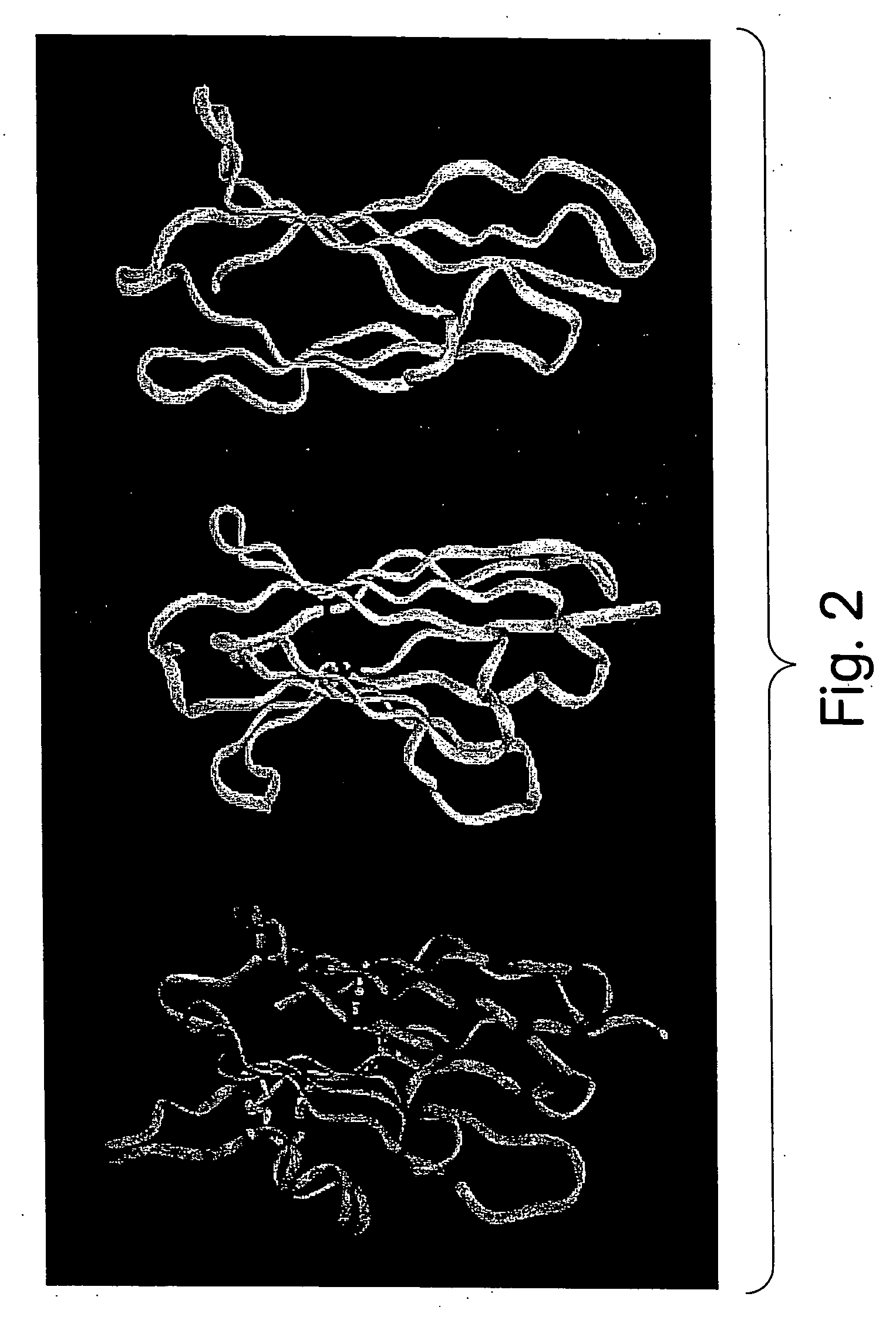
Protein scaffolds for antibody mimics and other binding proteins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0053] The novel antibody mimics described herein have been designed to be superior both to antibody-derived fragments and to non-antibody frameworks, for example, those frameworks described above.
[0054] The major advantage of these antibody mimics over antibody fragments is structural. These scaffolds are derived from whole, stable, and soluble structural modules found in human body fluid proteins. Consequently, they exhibit better folding and thermostability properties than antibody fragments, whose creation involves the removal of parts of the antibody native fold, often exposing amino acid residues that, in an intact antibody, would be buried in a hydrophobic environment, such as an interface between variable and constant domains. Exposure of such hydrophobic residues to solvent increases the likelihood of aggregation.
[0055] In addition, the antibody mimics described herein have no disulfide bonds, which have been reported to retard or prevent proper folding of antibody fragme...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com