Supercharged proteins for cell penetration
A supercharged, protein technology, applied in the field of supercharged proteins for cell penetration, can solve the problems of easy preparation, stability or universality of cytotoxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0475] Example 1: Supercharging a protein confers extraordinary resilience
[0476] Materials and methods
[0477] Design programs and supercharged protein sequences
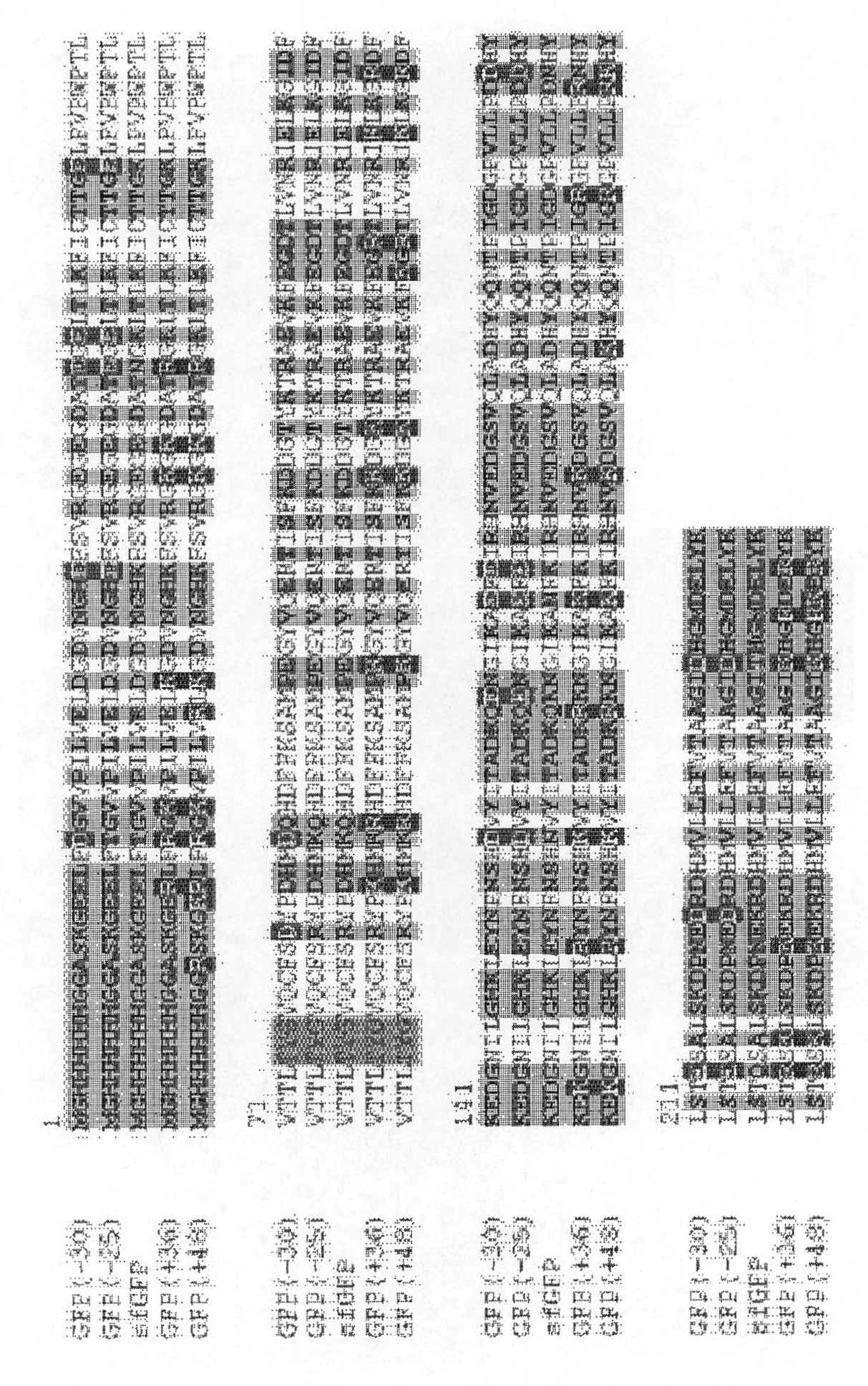
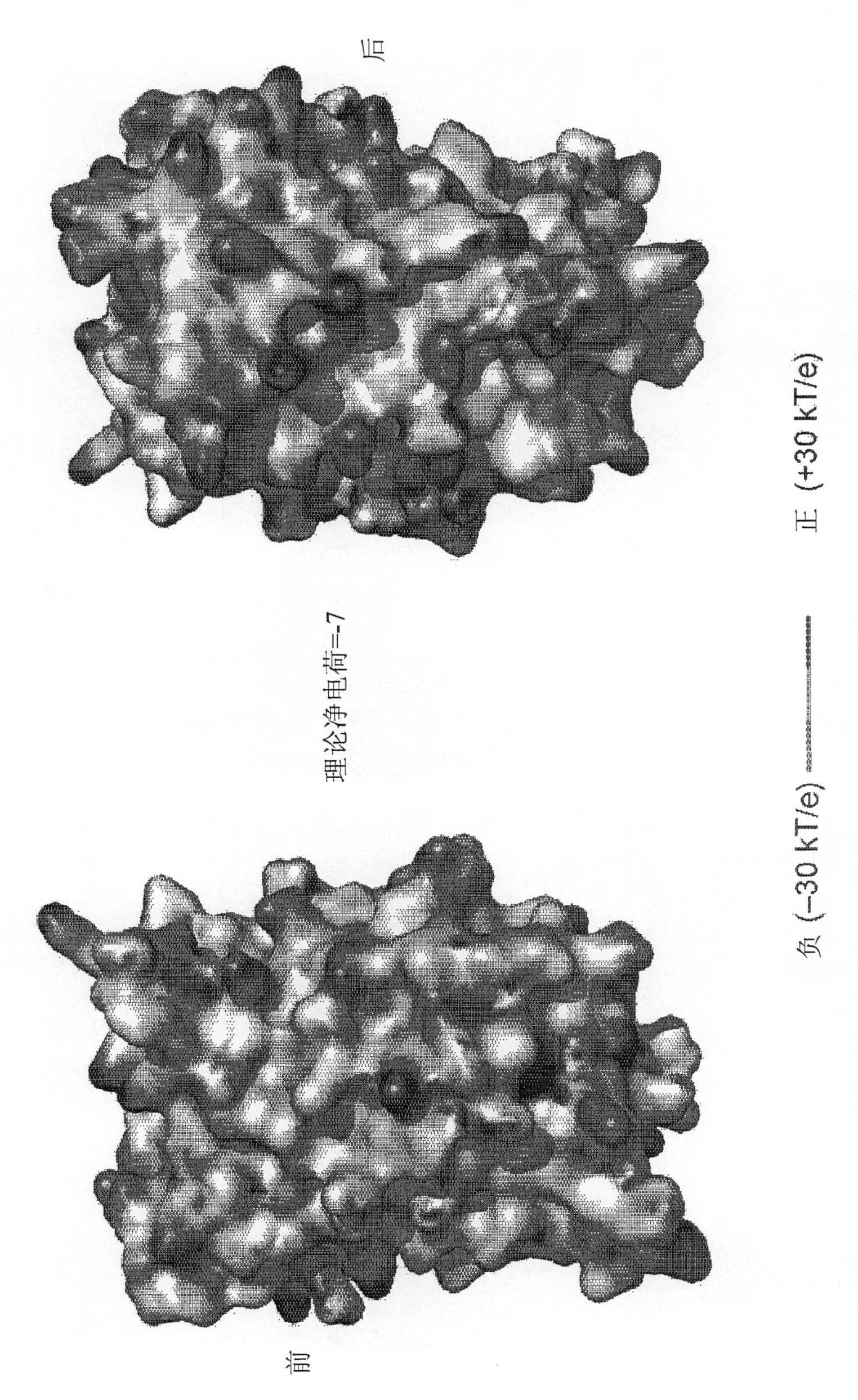
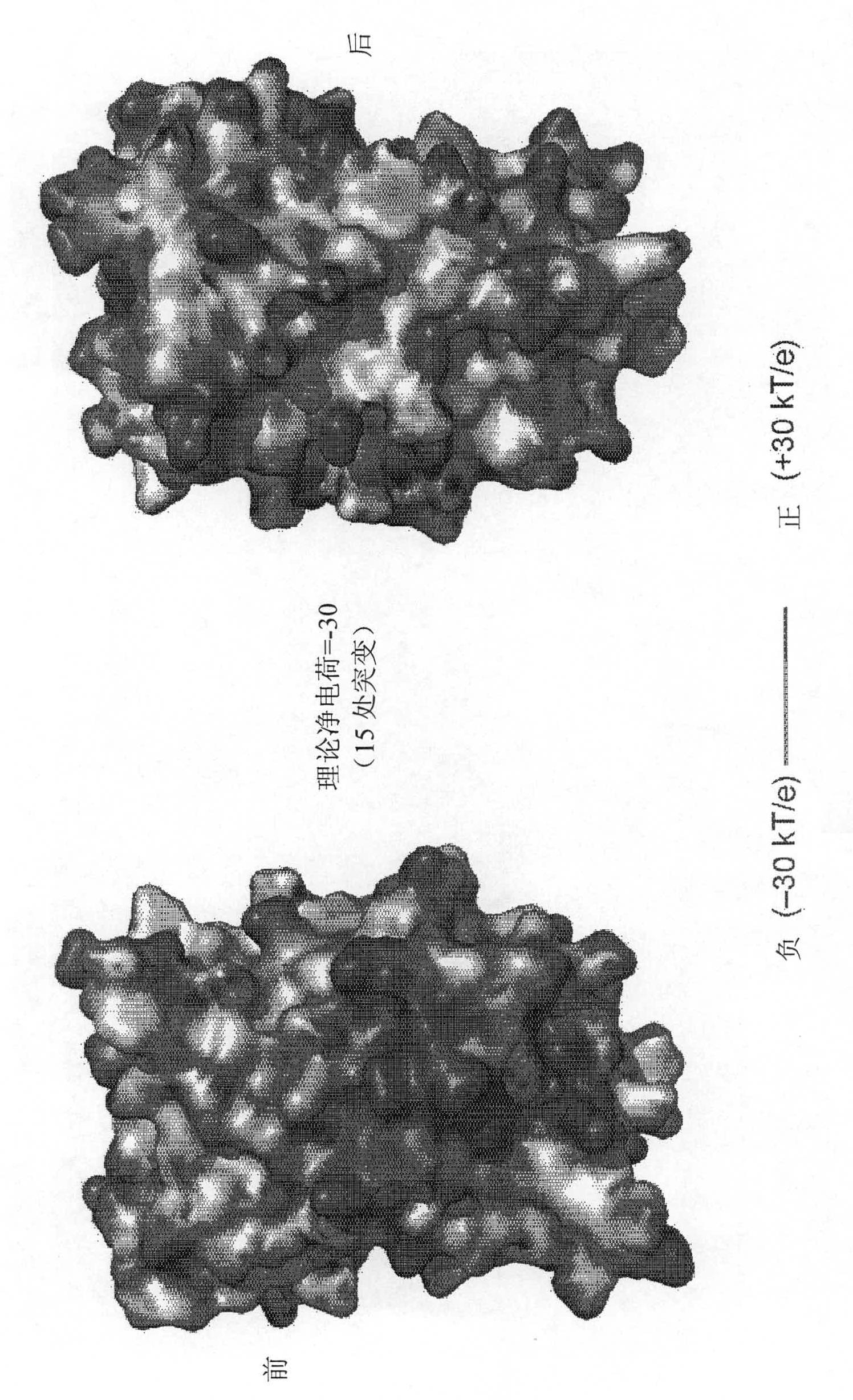
[0478] Solvent exposed residues of AvNAPSA Inside). A charged or highly polar solvent exposed residue (DERKNQ) was mutated to Asp or Glu for supernegative charge or to Lys or Arg for superpositive charge. Other surface-exposed positions to be mutated in green fluorescent protein (GFP) variants were selected based on the sequence variability of said positions among GFP homologs.
[0479] Protein Expression and Purification
[0480] Synthetic genes optimized for codon usage in E. coli were purchased from DNA 2.0, cloned into pET expression vector (Novegen) and overexpressed in E. coli BL21(DE3)pLysS at 15°C for 5-10 hours . Cells were harvested by centrifugation and lysed by sonication. Proteins were purified by Ni-NTA agarose chromatography (Qiagen), buffer exchanged to 100 mM NaCl, 50 mM potassium phosph...
example 2
[0516] Example 2: Supercharged proteins can be used to efficiently deliver nucleic acids into cells
[0517] Figure 5 showed that supercharged GFP can non-specifically and reversibly associate with oppositely charged macromolecules ("protein Velcro"). The interaction can lead to the formation of a precipitate. Unlike aggregates of denatured protein, these pellets contained folded fluorescent GFP and were soluble in 1M salt. Shown: +36GFP alone; +36GFP mixed with -30GFP; +36GFP mixed with tRNA; +36GFP mixed with tRNA in 1M NaCl; superfolded GFP ("sfGFP"; -7GFP); 30GFP mixed with sfGFP.
[0518] Image 6 shows that superpositively charged GFP binds siRNA. The binding stoichiometry between the two components was determined by mixing +36GFP with siRNA in different ratios (over 30 minutes at 25°C) and running the mixture on a 3% agarose gel (Kumar) et al., 2007, Nature, 449:39; which is incorporated herein by reference). The +36GFP:siRNA ratios tested were 0:1, 1:1, 1:2, 1:...
example 3
[0530] Example 3: Mammalian Cell Penetration, siRNA Transfection, and DNA Transfection of Supercharged Green Fluorescent Protein
[0531] Industry literature has recently described the resurfacing of proteins by extensive mutagenesis of non-conserved solvent-exposed residues without abolishing their structure or function (Lawrence MS, Phillips KJ, Liu DR (2007) , Supercharging proteins canimpart unusual resilience (Supercharging proteins canimpart unusual resilience), Journal of the American Chemical Society (J.Am.Chem.Soc.) 129: 10110-10112; applied on June 1, 2007 and served as WO 2007 / 143574 International PCT Patent Application PCT / US07 / 70254 published on December 13, 2007; U.S. Provisional Patent Application U.S.S.N. 60 / 810,364 filed on June 2, 2006 and Applied U.S.S.N. 60 / 836,607; each of which is incorporated herein by reference). When the replacement residues are all positively or all negatively charged, the resulting "supercharged" protein retains its activity while a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com