Type I boron fluoride complex dipyrromethene dye, and preparation method and application thereof
A technology of dipyrrole methine and boron fluoride, applied in the directions of methine-based/polymethine-based dyes, chemical instruments and methods, organic dyes, etc., can solve the problems of uneasy operation of reaction process, difficult synthesis of raw materials, low yield, etc. Good photostability, high molar extinction coefficient, simple reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
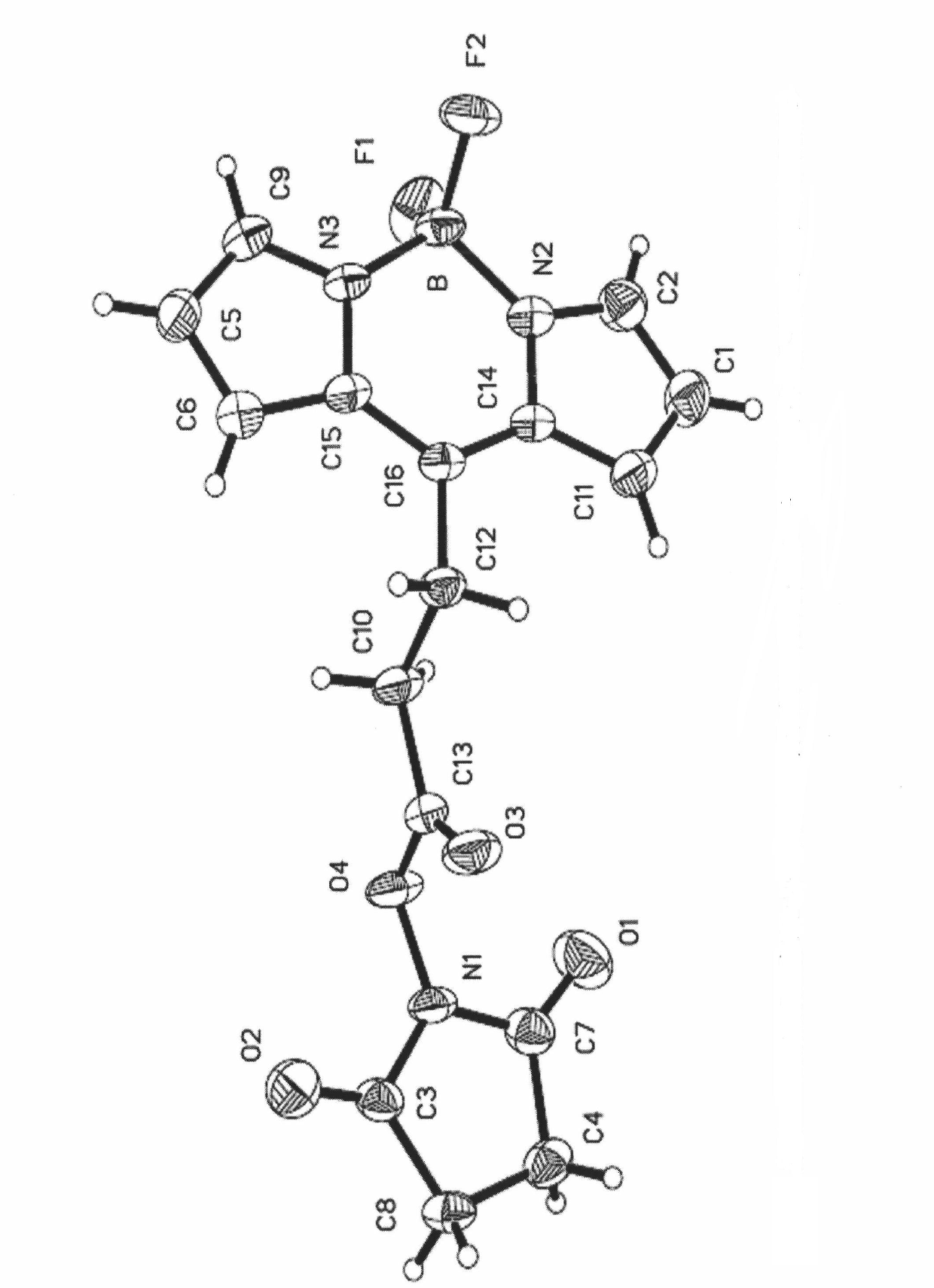
[0076] The preparation method of the present invention is different from the traditional synthetic method of using aldehydes or acid chloride compounds to react with substituted pyrroles to prepare dyes, but uses acid anhydrides to directly react with substituted pyrroles, and the synthetic method is improved and optimized, so that the yield Up to 25%.
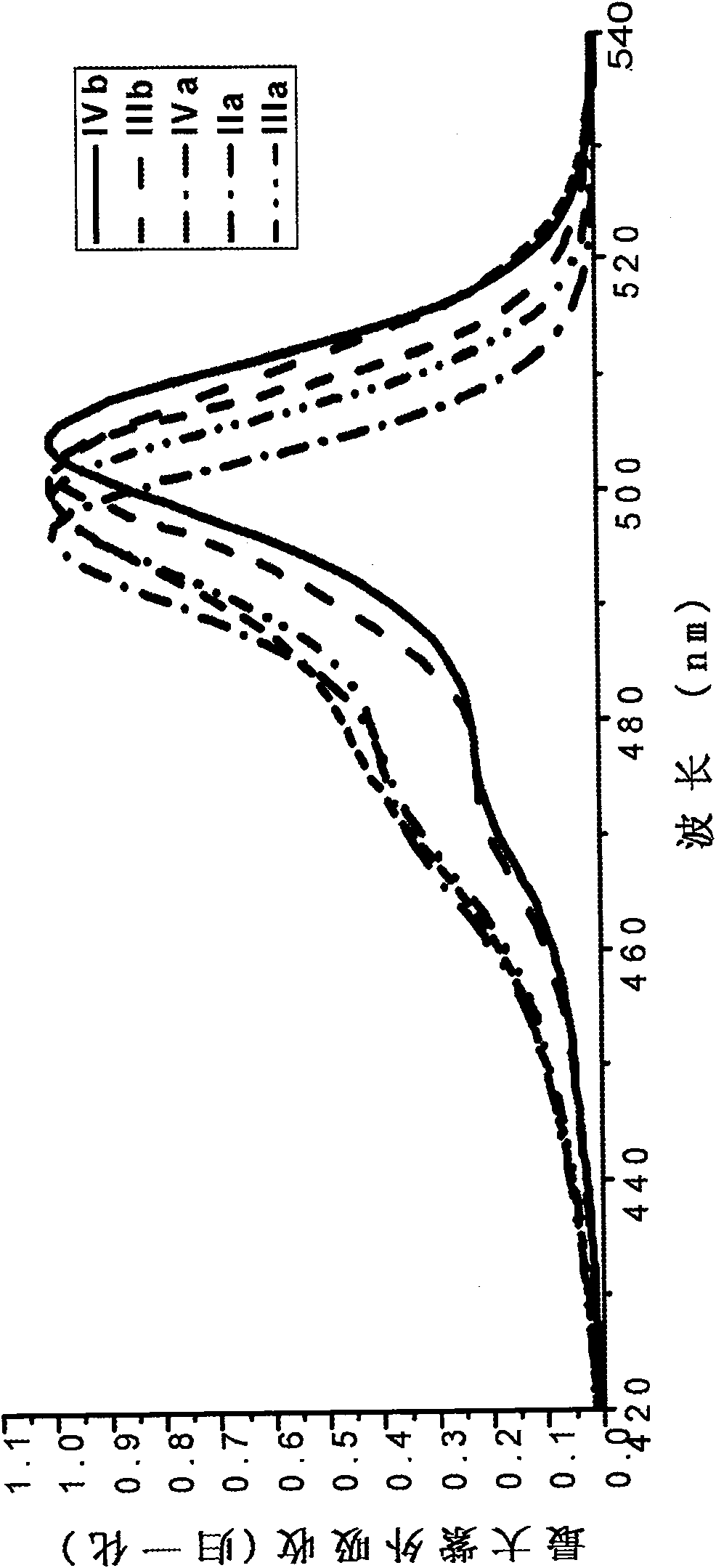
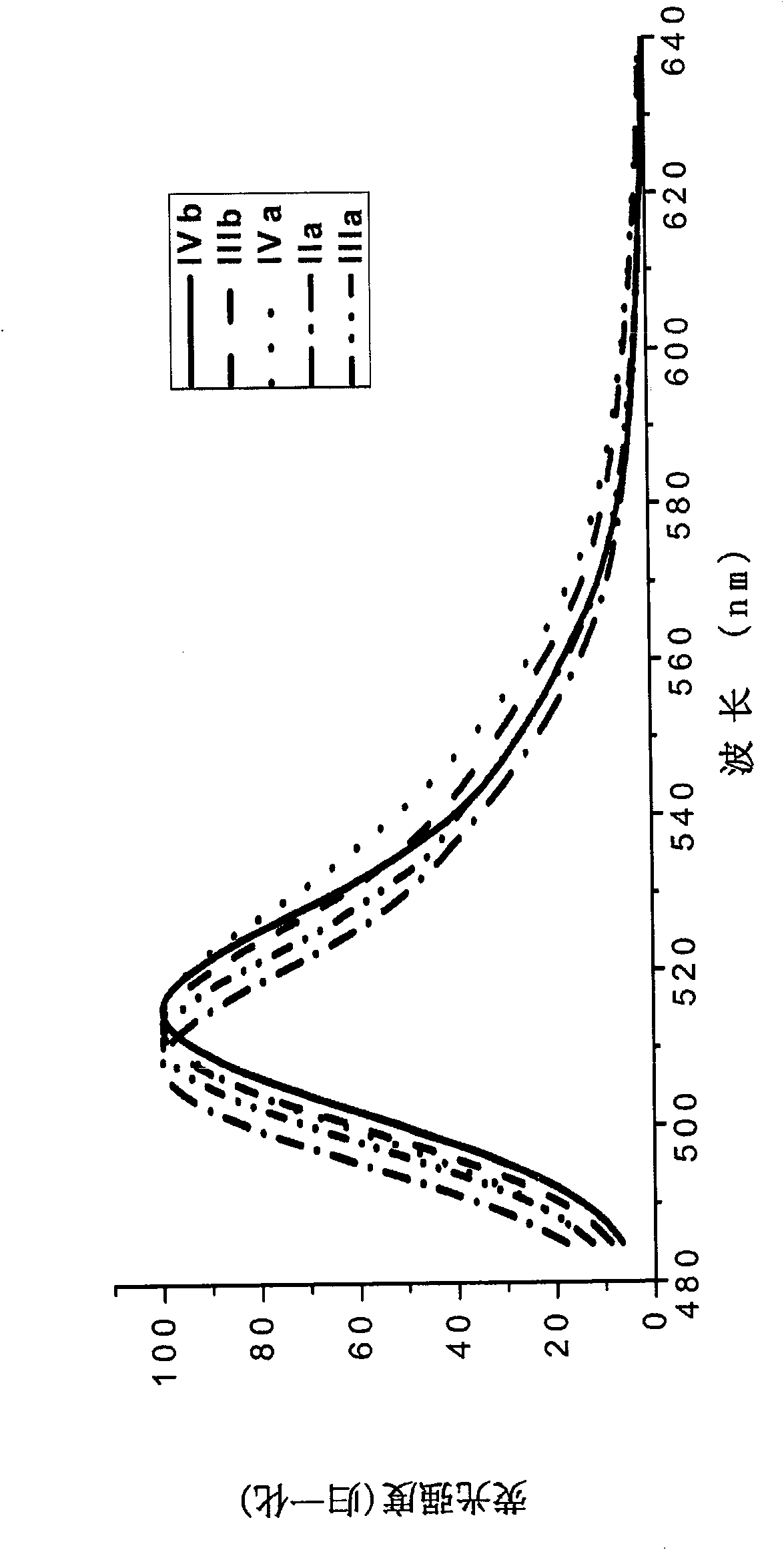
[0077] The boron fluoride complexed dipyrromethene fluorescent dye containing carboxylic acid and its derivatives of the present invention has a fluorescence quantum yield of 0.6-0.8, and the maximum ultraviolet absorption and fluorescence emission wavelengths are respectively at 495 ± 10nm and 510±10nm, the Stokes shift is between 10-20nm.
[0078] The boron fluoride complexed dipyrromethene fluorescent dye containing carboxylic acid and its derivatives of the present invention, when M in the fluorescent dye II, III or IV is a hydrogen proton, due to the presence of carboxylic acid groups, this type of dye is Low concentrati...
Embodiment 1
[0098] Synthesis of compound IIa:
[0099]
[0100] In the dry 250ml single-necked round bottom flask, add 204mg (1.8mmol) glutaric anhydride successively, 201mg (3mmol) pyrrole, then inject absolutely anhydrous fresh tetrahydrofuran 75ml with a syringe under nitrogen protection, control the reaction temperature as 55°C, magnetic stirring overnight, the color of the reaction solution is light brown; after cooling to room temperature and evaporating half of the solvent, slowly add 1.2g (12mmol) of anhydrous triethylamine under ice bath, after vigorously stirring for 10 minutes, drop Add 1.28g (9mmol) boron trifluoride ether solution. Keep stirring rapidly, and after the reaction solution returns to normal temperature, continue stirring for 4-8 hours. The whole reaction process was followed by TLC. After the reaction is complete, the mother liquor is poured into a separatory funnel, extracted with dichloromethane, washed with deionized water, and repeated three times, the o...
Embodiment 2
[0105] Synthesis of compound IIIa:
[0106]
[0107] In a 250mL single-necked round-bottom flask, add 160mg (1.6mmol) succinic anhydride and 201mg (3mmol) 1-hydropyrrole in sequence, then inject 10mL of dry acetonitrile and 50mL of DCM with a syringe under nitrogen protection, and stir rapidly with magnetic force to make the solid Dissolve (ultrasound or heating can also be used to promote dissolution), and then set up a condensing device and replace the reaction system with nitrogen for 3-5 times under vacuum, heat in an oil bath and reflux for overnight reaction; The mother liquor was cooled to room temperature, placed in an ice-water bath, and 2.4 g (24 mmol) of anhydrous triethylamine was slowly added dropwise. After vigorous stirring for 10 minutes, 2.56 g (18 mmol) of boron trifluoride ether solution was added dropwise. Keep stirring rapidly, after the reaction solution returns to normal temperature, slowly heat to 50°C, and continue stirring until no more fluorescent...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com