Method to control tumor progression and invasiveness
a tumor and invasiveness technology, applied in the field of biotechnology, can solve the problems of catenin-complex leading to invasion and metastasis, and achieve the effect of inducing invasion in vitro
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
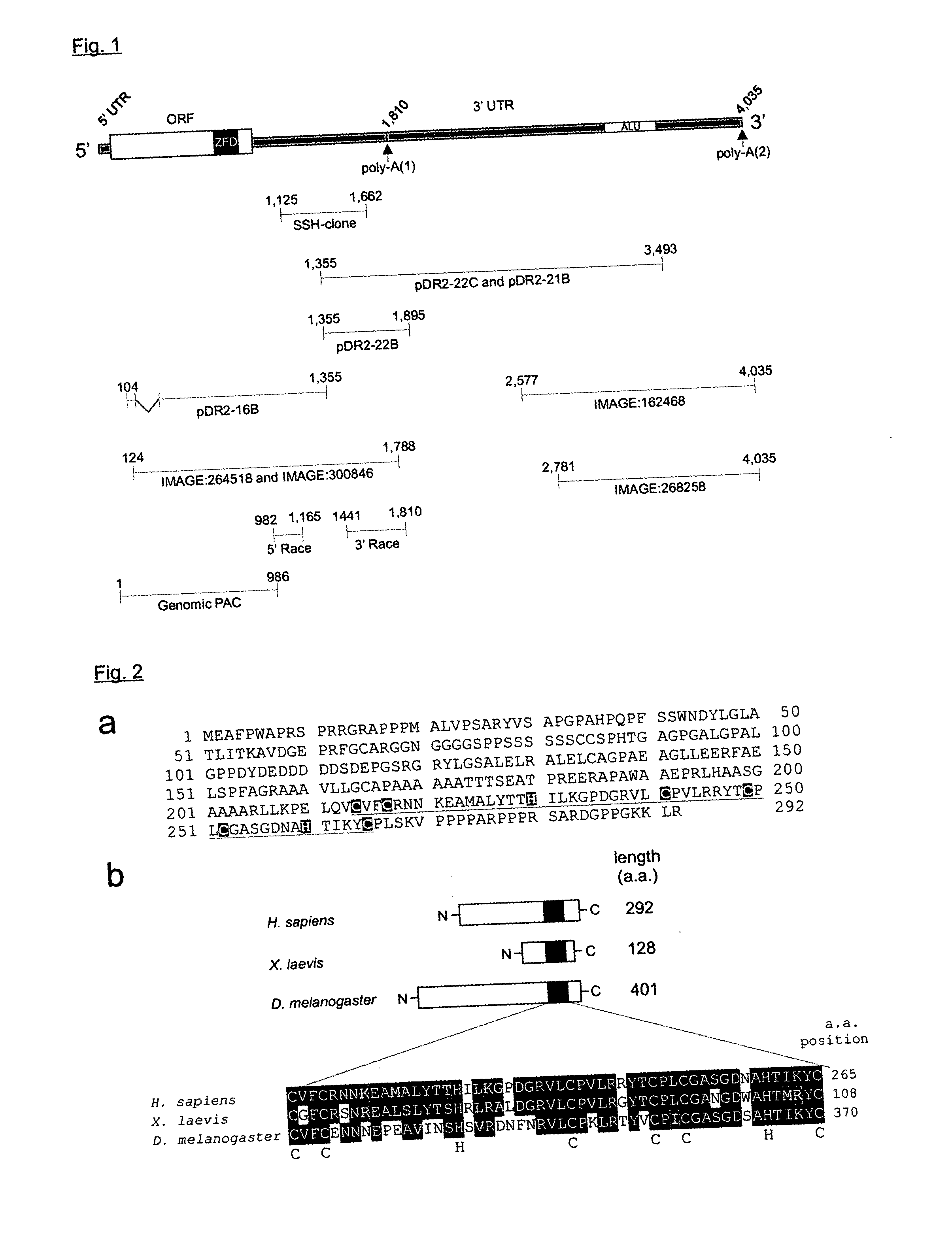
Isolation of the Full-Length hECRep1a cDNA Encoding a Human Nanos-Related Protein, and Several Homologs in Man and Mouse
E-Cadherin-Negative Versus -Positive Breast Carcinoma Cell Line System
[0069] A matched cell line couple, basically different in E-cadherin expression, was constructed by stable transfection of the invasive E-cadherin-negative cell line MDA-MB-231 with E-cadherin cDNA. This E-cadherin-positive MDA-MB-231-derivative, designated MDA2BE5.36, shows a restored epithelial phenotype in vitro with respect to strong and homogeneous expression of the E-cadherin / catenin-complex at the cell membrane, epithelioid cell morphology, growth repression, cadherin-dependent cell-cell aggregation, and a shift from a random towards a clustered spatial cell distribution, indicative for decreased invasiveness (Nawrocki-Raby et al., 2001).
Suppression Substractive Hybridization (SHH)
[0070] We have sought to identify genes whose expression is modulated by E-cadherin expression. To this ...
example 2
Expression Studies of hECRep1
hECRep1a mRNA Expression is Repressed by E-Cadherin Expression
[0076] The hECRep1a mRNA was found to be down regulated by E-cadherin expression in a Suppression Subtractive hybridization (SSH)-analysis of the E-cadherin-negative MDA-MB-231 breast cancer cell line versus an E-cadherin-transfected derivative of this cell line, MDA2BE5.36 The effective differential expression of hECRep1a in this cell line couple was confirmed by Northern blot analysis (FIG. 5a). Downregulation of hECRep1a mRNA expression by E-cadherin expression was further consolidated in SW480 cells, in which E-cadherin expression was induced by stable transfection of Smad4 cDNA (Schwarte-Waldhoff et al., 1999). Equal amounts of total RNA of both Smad4 transfected and mock transfected SW480 cells were analyzed by Northern blotting with an hECRep1a-specific probe (FIG. 5b). Smad4 transfected clones (D1 and D14) show induced E-cadherin mRNA expression and concomitantly reduced hECRep1a mR...
example 3
Human hECRep1a is a Single-Exon Gene Mapped to Chromosomal Region 10q25.3
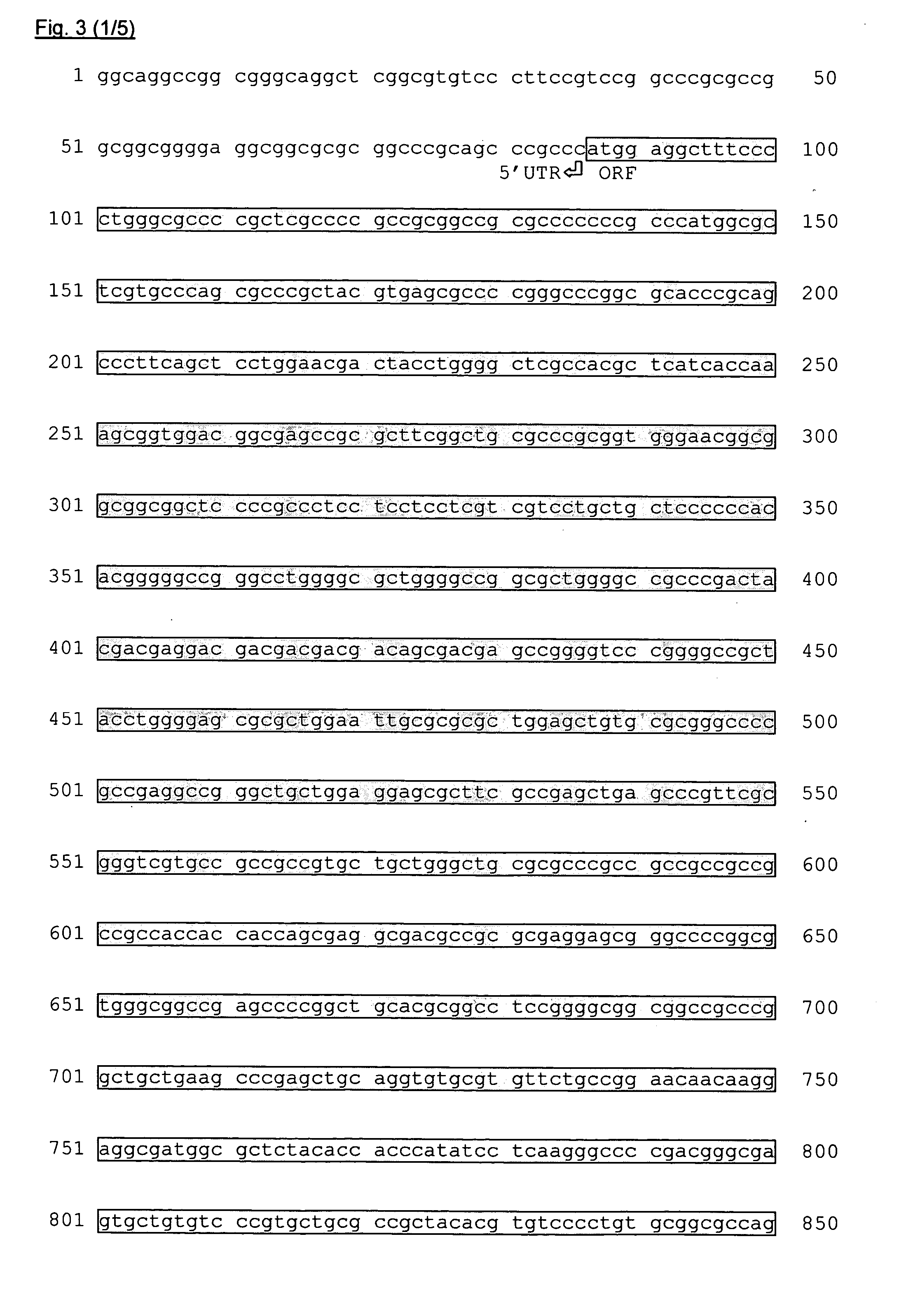
[0082] To investigate the genomic organization of the hECRep1 gene, a 12,650-nt genomic DNA contig, comprising the hECRep1a cDNA sequence, was subjected to an in silico analysis using the NIX tool at the UK HGMP resource centre (http: / / www.hgmp.mrc.ac.uk). The genomic contig contained 6,618 nt upstream and 1,997 nt downstream of the defined hECRep1a cDNA sequence. Based on the results of the GENSCAN analysis (Burge and Karlin, 1997), the hECRep1a gene was predicted to be a single-exon gene with an open reading frame as described above. Based on the GRAIL analysis (Xu et al., 1997), promoter elements were recognized including a TATA-box at nt position −33, a GC-box at nt position −54 and a CAAT element at nt position −278 relative to the predicted CAP-structure position (nt position 1 in the cDNA sequence). Reporter plasmids driven by different fragments of the hECRep1a promoter sequences were transiently trans...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Composition | aaaaa | aaaaa |
| Adhesion strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com