Separation and detection methods of circulating tumor cells
A technology of tumor cells and separation methods, which is applied in the separation and detection of circulating tumor cells, does not depend on the field of blood cell tumor markers, can solve the problem of indetermination of cell invasion ability, etc., and achieves the effect of simple separation and detection methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1 Detection of CTCs in mice transplanted with xenogeneic tumors
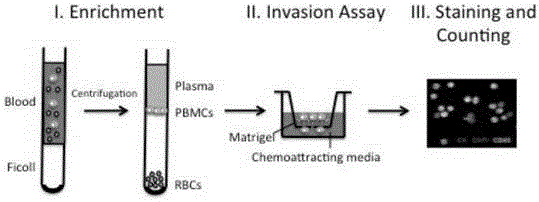
[0045]To develop a method for the isolation and detection of CTCs based on cell invasion ability, we first established a mouse model with metastatic tumors. A549, a non-small cell lung cancer cell line that stably expresses enhanced green fluorescent protein (GFP) and luciferase (Luc), was used to generate tumors in the flanks of nude mice. Surgical resection was performed when the flank tumors reached a diameter of 1 cm, and 60-70% of mice developed lung metastases 3 months after surgery.
[0046] Whole blood collection was performed 3 months after surgery. Whole blood was subjected to density gradient centrifugation using Ficoll lymphocyte separation medium to collect mononuclear cells (PBMCs), washed with serum-free RPMI-1640 cell culture medium, and PBMCs were resuspended.
[0047] The Transwell chamber invasion experiment was performed on the enriched PBMC: before the experiment, a layer of...
Embodiment 2
[0051] Example 2 Correlation between CTC detected by CICA and invasion and metastasis
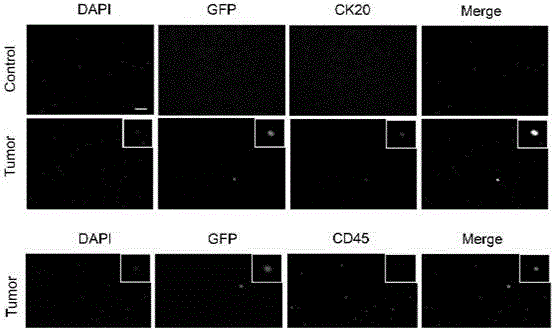
[0052] To demonstrate the utility of CICA, we tested CICA in a number of control and A549 xenografted nude mice. The whole blood of each transplanted tumor mouse and control mouse was collected, and CTC was detected by the method of CICA. We did not detect cytokeratin-positive CTCs in control mice. In contrast, blood samples from most tumor-implanted mice contained 22-78 CTCs positive for invasive GFP (Table 1). More interestingly, some mice transplanted with tumors had no detectable migrated CTCs (Table 1). These results indicated that CTCs isolated by CICA were associated with metastasis.
[0053] Table 1 CICA detection results of different mice
[0054]
Embodiment 3
[0055] Example 3 Comparison between CICA and EpCAM-based methods
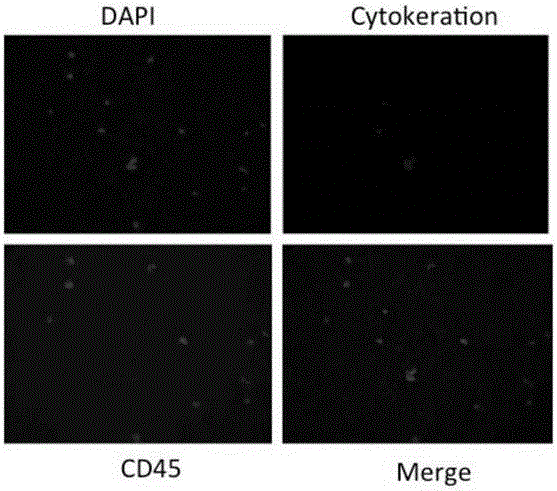
[0056] Currently, the most common isolation method for CTCs is immunomagnetic bead sorting using epithelial markers of CTCs. In order to compare these two methods, we divided the blood of mice transplanted with A549 tumor into two parts, and used these two methods to isolate CTCs respectively. The results showed that the EpCAM immunomagnetic bead method separated more than 40% of CTC cells than the CICA method (see Table 2).
[0057] However, when the CTCs screened by the immunomagnetic bead method were subjected to invasion experiments, only about 50% of the cells showed invasion ability. More interestingly, less invasive cells were screened by immunomagnetic beads than those isolated directly by CICA. Since immunomagnetic bead screening does not affect the invasive ability of cells, these results show that although more CTCs were obtained by anti-EpCAM antibody-based immune selection screening, many of them...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com