Peptoid and preparation method as well as application thereof
A type and reaction technology, applied in the field of medicine, can solve the problems of high inspection costs and not yet routinely carried out, and achieve the effects of low cost, rapid diagnosis, and simple synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] In this example, the peptoid was synthesized by solid-phase subunit synthesis, which specifically included the following steps:
[0059] (1) Rink amide AM resin (substitution level 0.3mmol / g) was swollen and deprotected with hexahydropyridine, and cysteine was mixed with 2-(3'-N-oxo-benzotriazole)-1, 1',3,3'-Tetramethyluronium hexafluorophosphate was equimolarly mixed, and coupled under the activation of N-methylmorpholine.
[0060] (2) Add 2M bromoacetic acid and 3.2M N,N'-diisopropylcarbodiimide (DIC) into Rink amideAM resin, react at 37°C for 30 minutes, and acylate the amino group at the end of the resin;
[0061] (3) Add 2M primary amine and react at 37°C for 90 minutes to replace the bromine atom through a nucleophilic substitution reaction to complete the synthesis of a subunit;
[0062] (4) Repeat steps (2) and (3) until completing the synthesis of the remaining units;
[0063] (5) After the synthesis is completed, the side chain protecting group is removed,...
Embodiment 2
[0067] In this example, the peptoid was synthesized by solid-phase subunit synthesis, which specifically included the following steps:
[0068] (1) Rink amide AM resin (substitution level 0.3mmol / g) was swollen and then deprotected with hexahydropyridine, and cysteine and O-benzotriazole-N,N,N',N'-tetra Methylurea tetrafluoroboric acid was mixed equimolarly and coupled under the activation of N-methylmorpholine.
[0069] (2) Add 2M bromoacetic acid and 3.2M N,N'-diisopropylcarbodiimide (DIC) to Rink amideAM resin, react at 25°C for 60 minutes, and acylate the amino group at the end of the resin;
[0070] (3) Add 2M primary amine and react at 25°C for 50 minutes to replace the bromine atom through a nucleophilic substitution reaction to complete the synthesis of a subunit;
[0071] (4) Repeat steps (2) and (3) until completing the synthesis of the remaining units;
[0072] (5) After the synthesis is completed, the side chain protecting group is removed, and the peptoid is c...
Embodiment 3
[0074] In this example, the peptoid was synthesized by solid-phase subunit synthesis, which specifically included the following steps:
[0075] (1) Rink amide AM resin (substitution level 0.3mmol / g) was swollen and then deprotected with hexahydropyridine, and cysteine and 1-hydroxybenzotriazole were mixed equimolarly, and in N-methylmorpholine Coupling was performed under activation.
[0076] (2) Add 2M bromoacetic acid and 3.2M dicyclohexylcarbodiimide to Rink amide AM resin, react at 40°C for 20 minutes, and acylate the amino group at the end of the resin;
[0077] (3) Add 2M primary amine and react at 20°C for 150 minutes to replace the bromine atom through a nucleophilic substitution reaction to complete the synthesis of a subunit;
[0078] (4) Repeat steps (2) and (3) until completing the synthesis of the remaining units;
[0079] (5) After the synthesis is completed, the side chain protecting group is removed, and the peptoid is cleaved from the resin with 95% triflu...
PUM
| Property | Measurement | Unit |
|---|---|---|
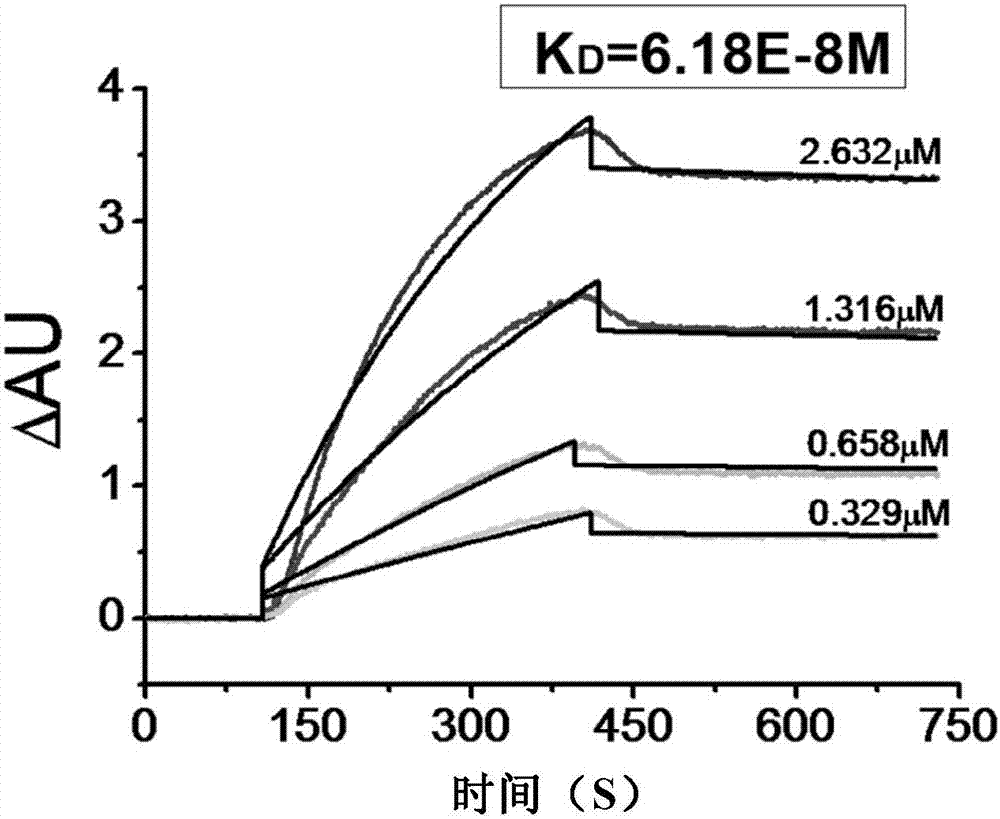
| Equilibrium dissociation constant | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com