Etoricoxib gel preparation and preparation method thereof
A gel preparation and etoricoxib technology, applied in the field of etoricoxib gel and preparation thereof, can solve problems such as poor solubility of etoricoxib, achieve stable and reliable preparation quality, simple production process and improved absorption rate Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
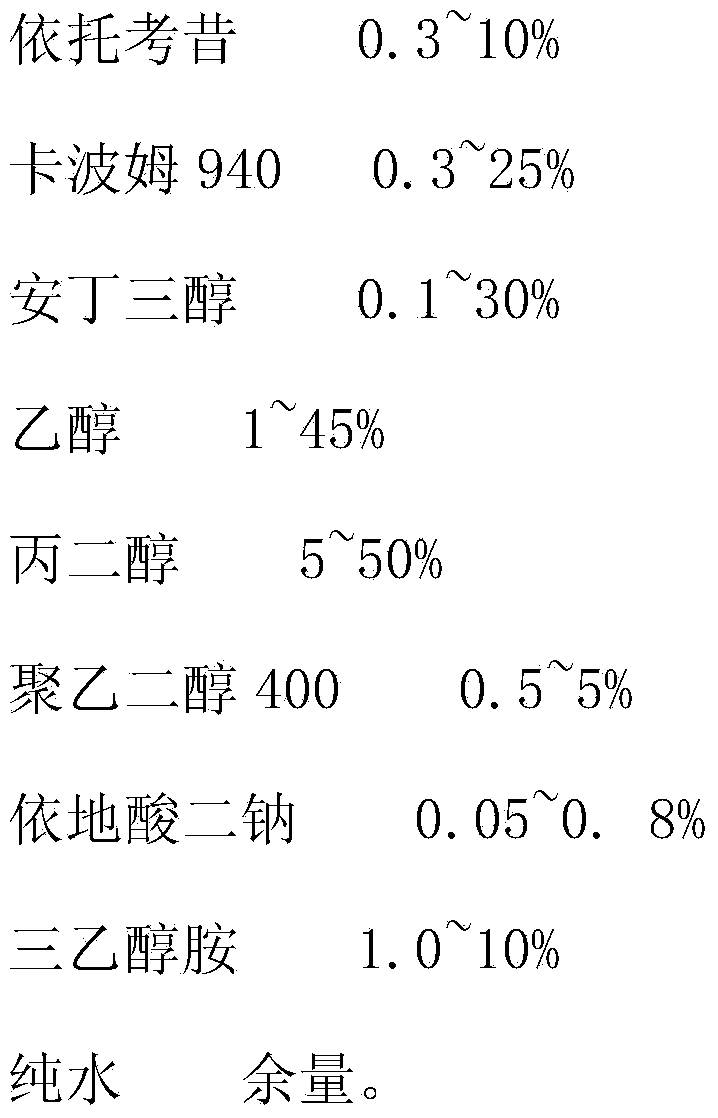
[0028]
[0029]
[0030] Preparation process
[0031] Put the carbomer in purified water with 50% of the total prescription and soak for 24 hours. The tromethamine is dissolved in water with 10% of the total dosage of the prescription for subsequent use. Put etoricoxib in ethanol and stir to disperse evenly, add tromethamine aqueous solution and stir to dissolve until the solution is completely clear, and set aside. Stir and dissolve edetate disodium and the remaining purified water until there are no visible particles, and set aside; filter the swollen carbomer 940 through 120 mesh, and add the prescribed amount of propylene glycol, polyethylene glycol 400, edetate di Sodium solution and etoricoxib solution are added to Carbomer. Stir for 15 minutes; add triethanolamine into the carbomer matrix, stir for 30 minutes, control the pH at 7.3-8.5, stop stirring, fill, pack, and obtain etoricoxib gel.
Embodiment 2
[0033] The etoricoxib raw material is formulated into etoricoxib gel according to the following prescription:
[0034]
[0035]
[0036]Put the carbomer in purified water with 50% of the total prescription and soak for 24 hours. The tromethamine is dissolved in water with 10% of the total dosage of the prescription for subsequent use. Put etoricoxib in ethanol and stir to disperse evenly, add tromethamine aqueous solution and stir to dissolve until the solution is completely clear, and set aside. Stir and dissolve edetate disodium and the remaining purified water until there are no visible particles, and set aside; filter the swollen carbomer 940 through 120 mesh, and add the prescribed amount of propylene glycol, polyethylene glycol 400, edetate di Sodium solution and etoricoxib solution are added to Carbomer. Stir for 15 minutes; add triethanolamine into the carbomer matrix, stir for 30 minutes, control the pH at 7.3-8.5, stop stirring, fill, pack, and obtain etorico...
Embodiment 3
[0038] The etoricoxib raw material is formulated into etoricoxib gel according to the following prescription:
[0039]
[0040] Put the carbomer in purified water with 50% of the total prescription and soak for 24 hours. The tromethamine is dissolved in water with 10% of the total dosage of the prescription for subsequent use. Put etoricoxib in ethanol and stir to disperse evenly, add tromethamine aqueous solution and stir to dissolve until the solution is completely clear, and set aside. Stir and dissolve edetate disodium and the remaining purified water until there are no visible particles, and set aside; filter the swollen carbomer 940 through 120 mesh, and add the prescribed amount of propylene glycol, polyethylene glycol 400, edetate di Sodium solution and etoricoxib solution are added to Carbomer. Stir for 15 minutes; add triethanolamine into the carbomer matrix, stir for 30 minutes, control the pH at 7.3-8.5, stop stirring, fill, pack, and obtain etoricoxib gel.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com