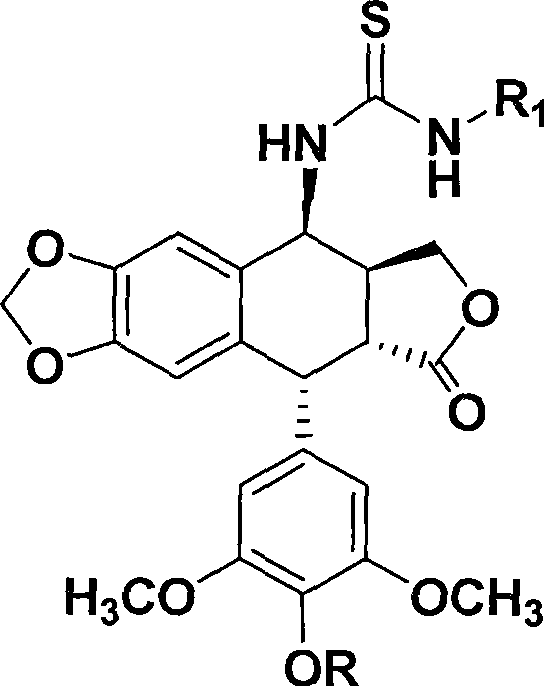
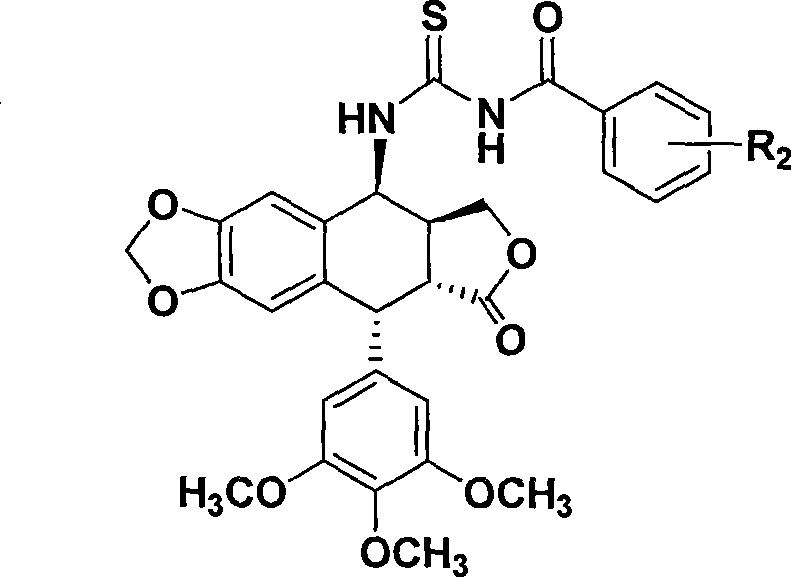
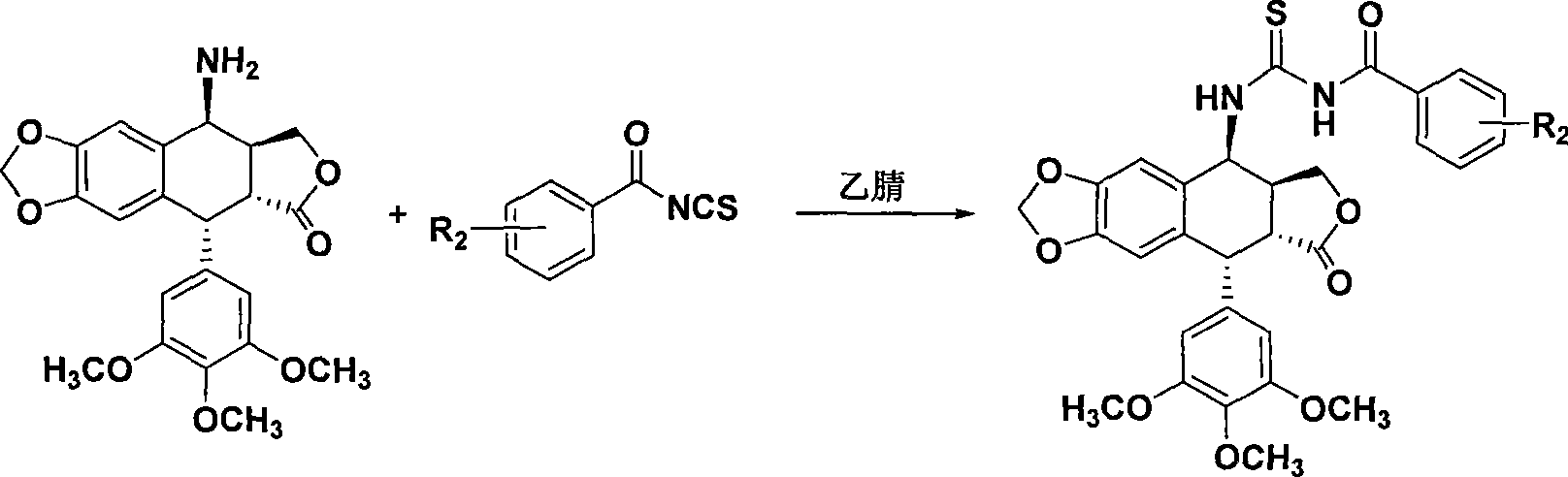
Thiourea homologues epipodophyllotoxin compound and preparation method and application thereof
A technology of epipodophyllotoxin and compounds, applied in the fields of medicinal chemistry and pharmacology, can solve the problems of low selectivity and large side effects, and achieve the effect of simple preparation method, easy operation, and inhibitory activity of tumor cell growth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1: Compound 2a: 4-[Benzoylthioureido]epipodophyllotoxin
[0028]
[0029] Add benzoyl isothiocyanate (195mg, 1.2mmol) to acetonitrile (20mL), add 4-aminoepipodophyllotoxin (413mg, 1mmol), reflux reaction for 4h, TLC detects that the reaction is complete, concentrate under reduced pressure, and use a silica gel column Chromatography (ethyl acetate:petroleum ether, 1:3) afforded 2a (446 mg, 77.5%) as a white solid.
[0030] R f 0.25 (ethyl acetate: petroleum ether, 1:3);
[0031] 1 H NMR (CDCl 3 ): δ2.00(s, 1H, 1-NH), 2.93(dd, J=4.8, 14.4, 1H, 2-H), 3.07(m, 1H, 3-H), 3.73(s, 6H, 3 ',5'-OCH 3 ), 3.78 (s, 3H, 4'-OCH 3 ), 3.80 (d, 1H, 4-H), 4.32 (m, 2H, 11-H), 4.62 (d, J=4.8, 1H, 1-H), 5.96 (s, 2H, -OCH 2 O), 6.28 (s, 2H, 2′, 6′-ArH), 6.52 (s, 1H, 8-ArH), 6.84 (s, 1H, 5-ArH), 7.48 (d, J=7.5, 1H, 3″-ArH), 7.51 (d, J=5.7, 1H, 5″-ArH), 7.62 (t, J=5.7, 1H, 4″-ArH), 7.79 (s, 1H, 2″-ArH), 7.81 (s, 1H, 6″-ArH), 10.67 (d, J=7.8, 1H, 2-NH);
[0032] 13 C NMR (C...
Embodiment 2
[0068] Drug Example 2: Cytotoxic Activity of Compound 2b on HepG-2 Cells
[0069] Cells in the logarithmic growth phase were inoculated on a 96-well plate, and drugs of different concentrations were added after 24 hours of adherence. Four parallel wells were set up for each concentration. After 68 hours of culture, MTT solution was added, and the culture was continued for 4 hours. The culture medium was discarded, and 150 μL of DMSO was added. Shake for 10min, measure the absorbance (A) value at 570nm with a microplate reader, and calculate the half inhibitory concentration (IC 50 ). IC of compound 2b 50 3.2×10 -5 M, while the IC of the positive control etoposide on HepG-2 cells 50 4.5×10 -4 M.
[0070] Experimental conclusions The thiourea epipodophyllotoxin with the structure shown in formula (1) has strong cytotoxic activity on HepG-2 cells, and may be developed into a new drug with anti-tumor effect.
Embodiment 3
[0071] Drug Example 3: Cytotoxic activity of compound 2c on Hela cells
[0072] Cells in the logarithmic growth phase were inoculated on a 96-well plate, and drugs of different concentrations were added after 24 hours of adherence. Four parallel wells were set up for each concentration. After 68 hours of culture, MTT solution was added, and the culture was continued for 4 hours. The culture medium was discarded, and 150 μL of DMSO was added. Shake for 10min, measure the absorbance (A) value at 570nm with a microplate reader, and calculate the half inhibitory concentration (IC 50 ). IC of compound 2-5 50 4.2×10 -5 M, while the IC of the positive control etoposide on Hela cells 50 5.0×10 -5 M.
[0073] Experimental conclusion The thiourea epipodophyllotoxin with the structure shown in formula (1) has strong cytotoxic activity on Hela cells, and may be developed into a new drug with antitumor effect.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com