Application of JAM-A (junctional adhesion molecules A) genes or protein in genetic recombination drugs for treating rheumatoid arthritis
A JAM-A, gene recombination technology, applied in the direction of drug combination, microbial determination/examination, antipyretic drugs, etc., can solve the problems of lack of clinical treatment methods, complex pathogenesis, adverse reactions of patients, etc., and achieve simple production process, The effect of short production cycle and easy expansion of large-scale production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
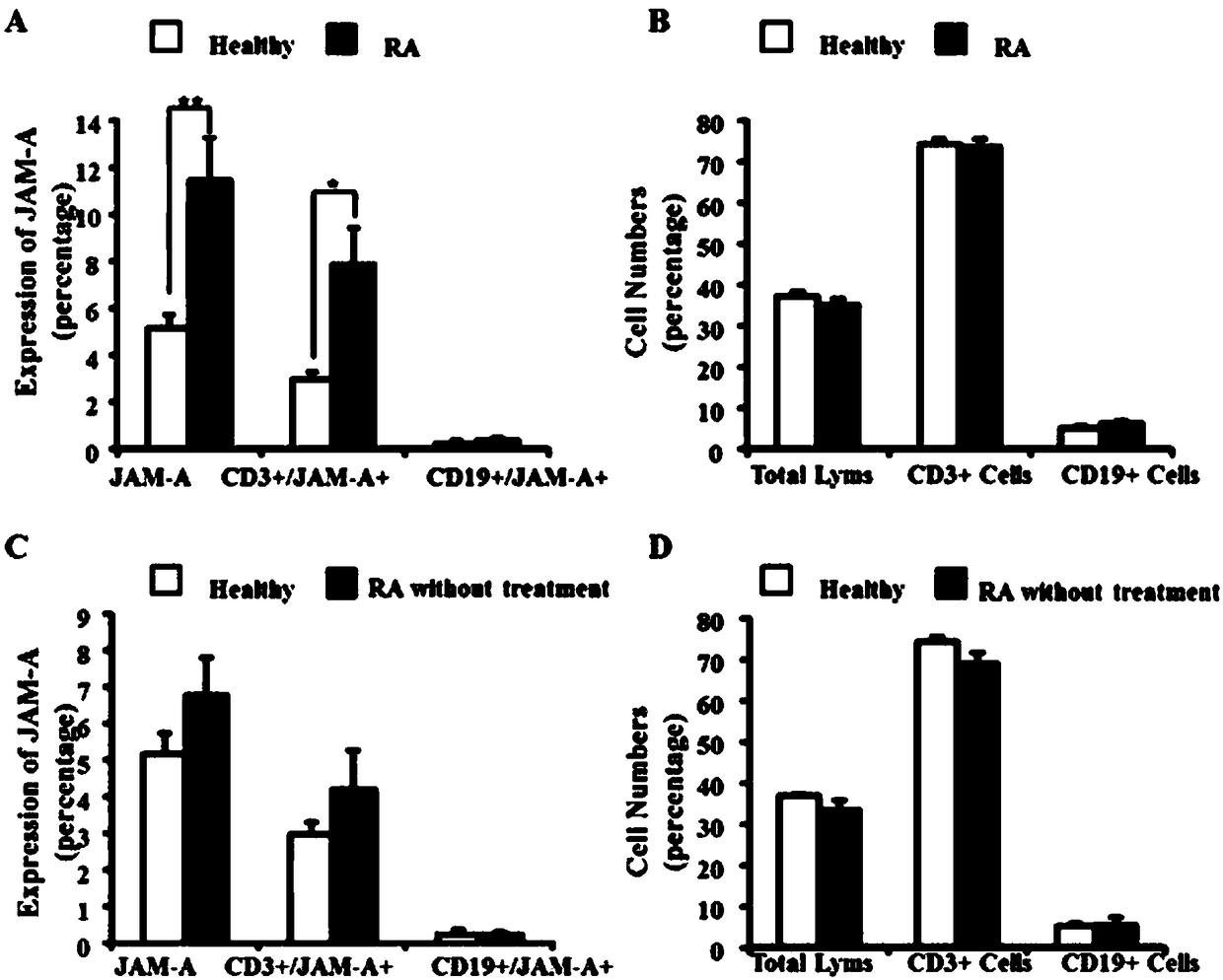
[0013] Example 1: Expression of JAM-A protein in peripheral blood lymphocytes of healthy people and RA patients.
[0014] 1. Collection and grouping of blood samples.
[0015] The peripheral blood sample collected in the present invention informs volunteers and patients with rheumatoid arthritis of the experimental purpose before blood collection and signs an informed consent. review.
[0016] The present invention collected peripheral blood from 48 patients with rheumatoid arthritis (rheumatoid arthritis group, RA), all from the Department of Rheumatology and Immunology, the First Affiliated Hospital of Kunming Medical University. The peripheral blood of 37 healthy adult volunteers (normal group, CON) was collected from the First Affiliated Hospital of Kunming Medical University and volunteers of Yunnan University. Each patient and volunteer was asked about the medical history by two attending physicians in the department of rheumatology and immunology and underwent a speci...
Embodiment 2
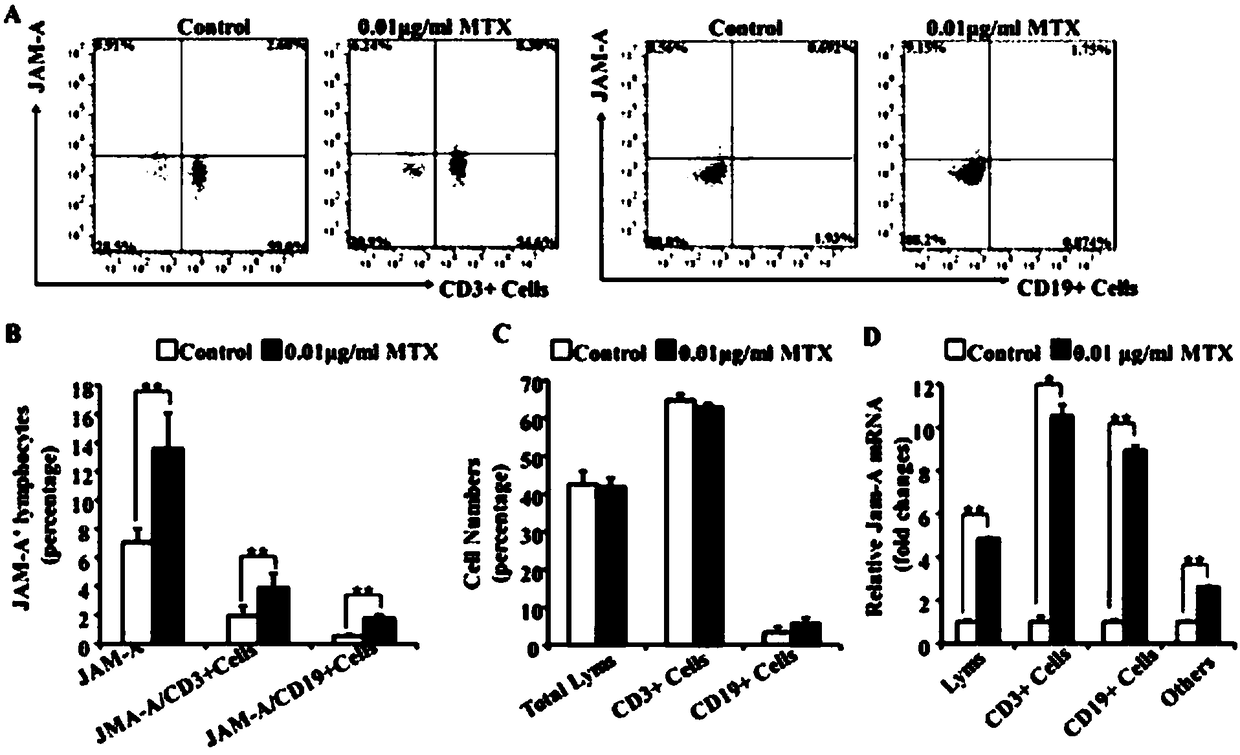
[0019] Example 2: Effect of JAM-A expression on lymphocyte migration ability.
[0020] 1. Common rheumatoid arthritis treatment drugs can effectively increase the expression of junctional adhesion molecule A.
[0021] Lymphocytes treated with 0.01 μg / mL methotrexate for 5 days were collected, total RNA was extracted and extracted using Takara’s PrimeScript RT reagent Kit with gDNA Eraser (Perfect Real Time) (Takara, Code No.DRR047A) kit. RNA is reverse transcribed into cDNA. The expression of junctional adhesion molecule A was analyzed by fluorescent quantitative PCR with the following primers:
[0022] Human junction adhesion molecule A (301-409, 109bp):
[0023] Forward: 5'- CGGGAAGACACTGGGACATA- 3';
[0024] Reverse: 5'-CTGTAGGCTTGGATGGAGGC-3';
[0025] Human β-actin (939-1143, 205bp):
[0026] Forward: 5'- TGACGTGGACATCCGCAAAG-3';
[0027] Reverse: 5'-CTGGAAGGTGGACAGCGAGG-3'.
[0028] The amplification program was pre-denaturation at 94°C for 3 min; 36 cycles of 30 ...
Embodiment 3
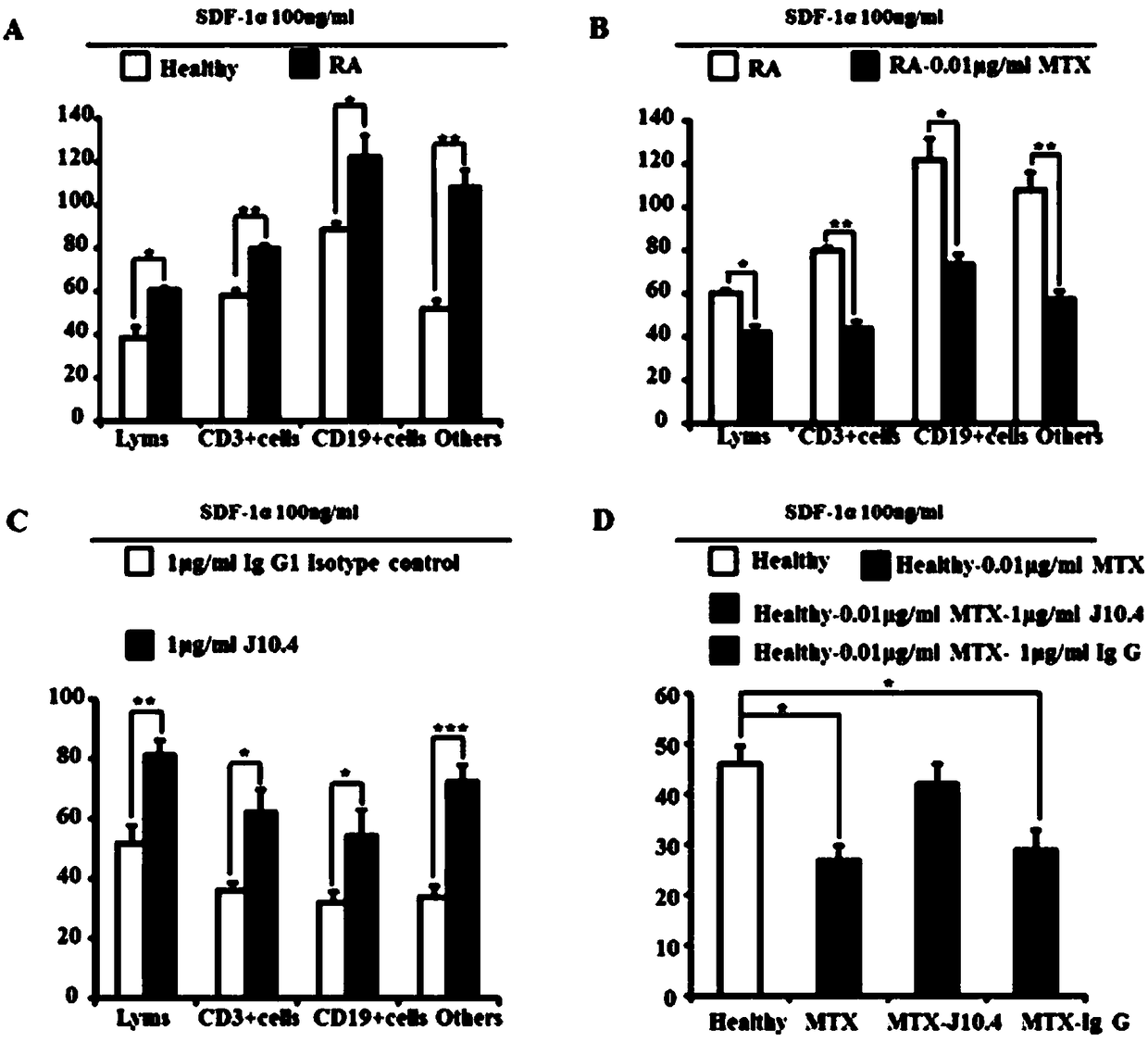
[0031] Example 3: Treatment of Collagen-Induced Arthritis (CIA) Mice with Adhesion Molecule A Linked Neutralizing Antibody J10.4.
[0032] 1. Grouping of experimental mice.
[0033] Select 6-8 weeks and C57BL / 6 female mice with a body weight of 22-26 g (Beijing Weitong Lihua Company, clean grade), and intradermally immunize bovine type II collagen at the base of the tail to establish a CIA model, and the time of mouse immunization Defined as Day 0 (Day 0). 8 normal control groups (Control group, CON) and 24 diseased mice were randomly divided into three groups. The first group was the CIA model group without any treatment; the second group was the junction adhesion molecule A specific antibody J10.4 To treat the CIA model group, at the peak of CIA, that is, 20 days after collagen immunization, 1 mg / kg of J10.4 neutralizing antibody was injected into the tail vein every other day for 10 consecutive days. The third group is the IgG negative control group, the immunization time...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com