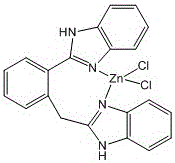
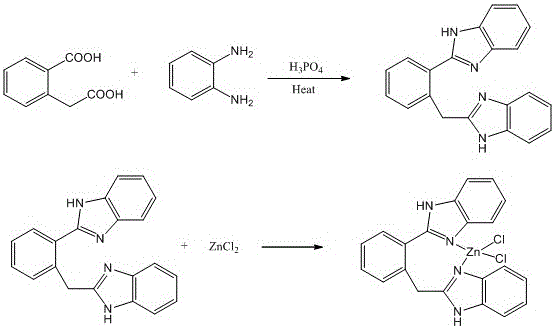
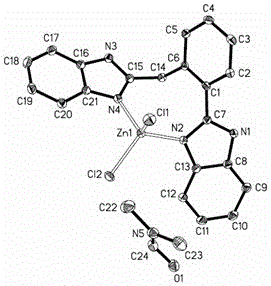
A kind of benzimidazole zinc complex and preparation method thereof
A technology of benzimidazole zinc and benzimidazole, applied in the field of metal complex antitumor drugs, can solve the problems of difficult to meet the clinical needs of esophageal cancer treatment, many adverse reactions, easy to produce drug resistance, etc., and achieve the purpose of inhibiting esophageal cancer cells Ease of growth and post-treatment, good inhibitory effect on the growth and activity of esophageal cancer cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] (1) Ligand 2-(2-(1H-benzimidazole-2-)benzyl)-1H-benzimidazole synthesis method
[0026] 2-(Carboxymethyl)benzoic acid (1.80g, 0.010mol) and o-phenylenediamine (2.16g, 0.020mmol) were mixed in phosphoric acid (20mL), heated at 180°C for 3 hours, and the reactant was cooled to room temperature, then Distilled water was added and a yellow solid was obtained after neutralization with ammonia water. Washed 3 times with distilled water, then filtered, and recrystallized from ethanol to obtain yellow-green solid 2-(2-(1H-benzimidazole-2-)benzyl)-1H-benzimidazole, the yield was 2.16g, and the yield was 62.4 %.
[0027] Elemental Analysis: C 21 h 20 N 4 o 2 (bbb·CH 3 CH 2 OH·H 2 O), the theoretically calculated value is C, 71.11%; H, 6.23%; N, 14.42%, the experimental value is C, 71.48%; H, 6.I1%; N, 14.33%; Infrared spectrum: IR (KBr, cm -1 ): 3406, 3058, 1627, 1534, 1437, 1379, 1310, 1271, 1155, 839, 799, 724; Proton NMR spectrum: 1 HNMR: (300MHz, CDCl 3 ):1.26(m, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com