Eremophil dilactone with tumour cell growth inhibiting activity and its use
A technology of lactone and cancer cells, applied in the field of natural medicinal chemistry and pharmacology, can solve the problems that the research of biological activity has not been reported yet
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
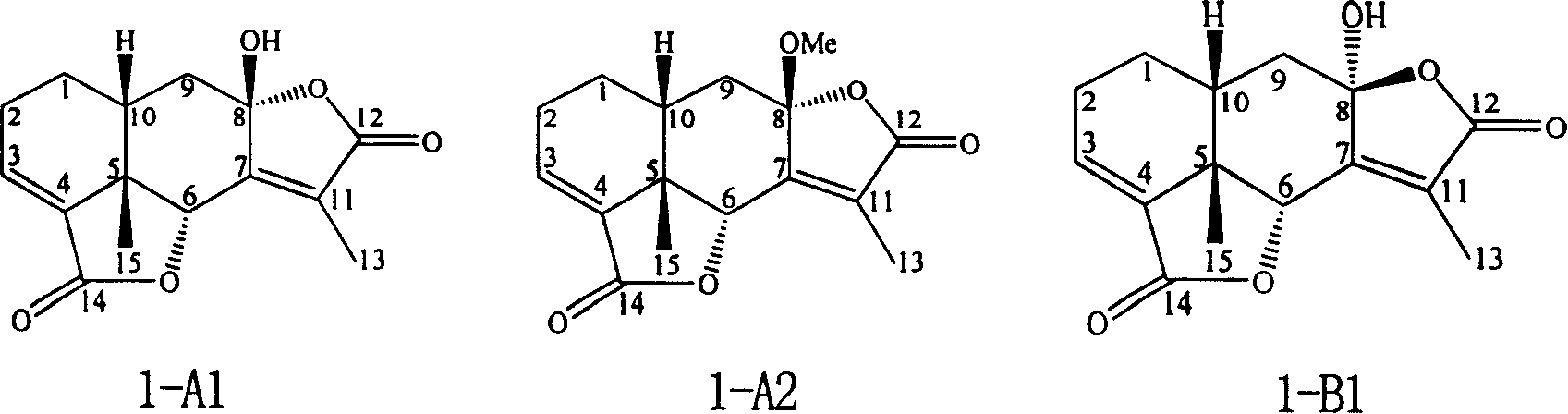
[0026] Example 1: Preparation of Compound 1-A1
[0027] Take 5.0 kg of dry underground part of Ligularia atroviblacea (Franch.) Hand.-Mazz., pulverize into powder, add 50 liters of 95% ethanol to soak. Soak 3 times at room temperature, 7 days each time. The ethanol extracts were combined, and the solvent was recovered under reduced pressure to dryness to obtain 462 grams of extract, dispersed with 3 liters of water, and then extracted with 60-90° petroleum ether, ethyl acetate, and n-butanol in sequence. The ethyl acetate extract was recovered under reduced pressure to obtain 89.0 g of extract. The extract was chromatographed on 800 g of 100-200 mesh silica gel column, and chloroform-methanol (50:0-0:1) gradient eluted. Thin-layer chromatography detection combined the same fractions, and the part of the extract containing compound 1-A1 was purified by preparative thin layer (60-90° petroleum ether-ethyl acetate 4:1) followed by Sephadex LH-20 column chromatography ( elutin...
Embodiment 2
[0029] Example 2: Preparation of compound 1-A2
[0030] With embodiment 1, obtain 89.0 gram ethyl acetate extracts from 5.0 kilograms of drying Heiziwu. The extract was chromatographed on 800 g of 100-200 mesh silica gel column, and chloroform-methanol (50:0-0:1) gradient eluted. Thin-layer chromatography detection merged the same fractions, and the partial extract (4.8 grams) containing compound 1-A2 was subjected to 200-300 mesh silica gel (90 grams) column chromatography, and was mixed with 60-90° petroleum ether-ethyl acetate 8 :1-2:1 gradient elution, followed by preparative TLC (60-90° petroleum ether-ethyl acetate 6:1) purification to finally obtain compound 1-A2 (17 mg).
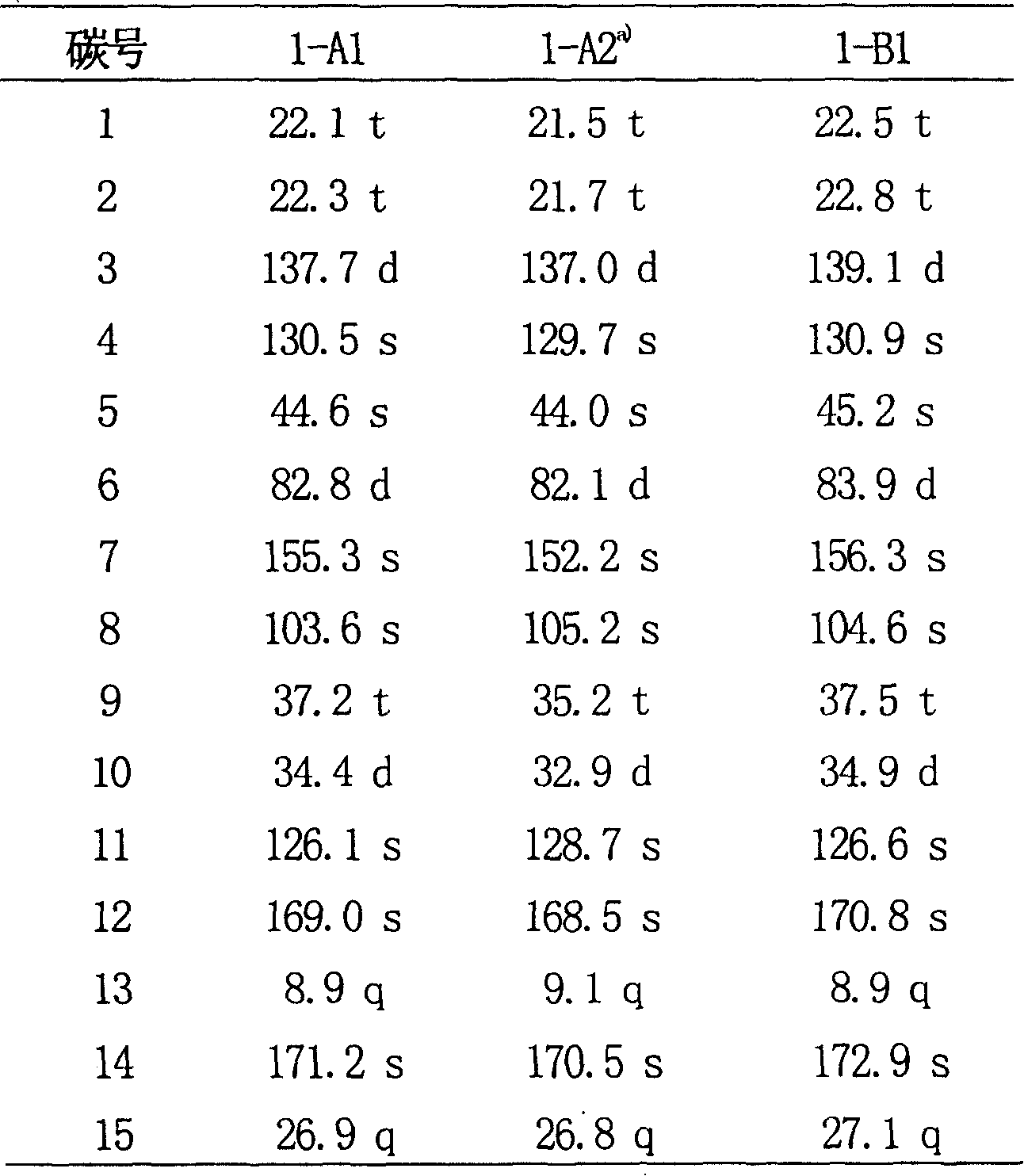
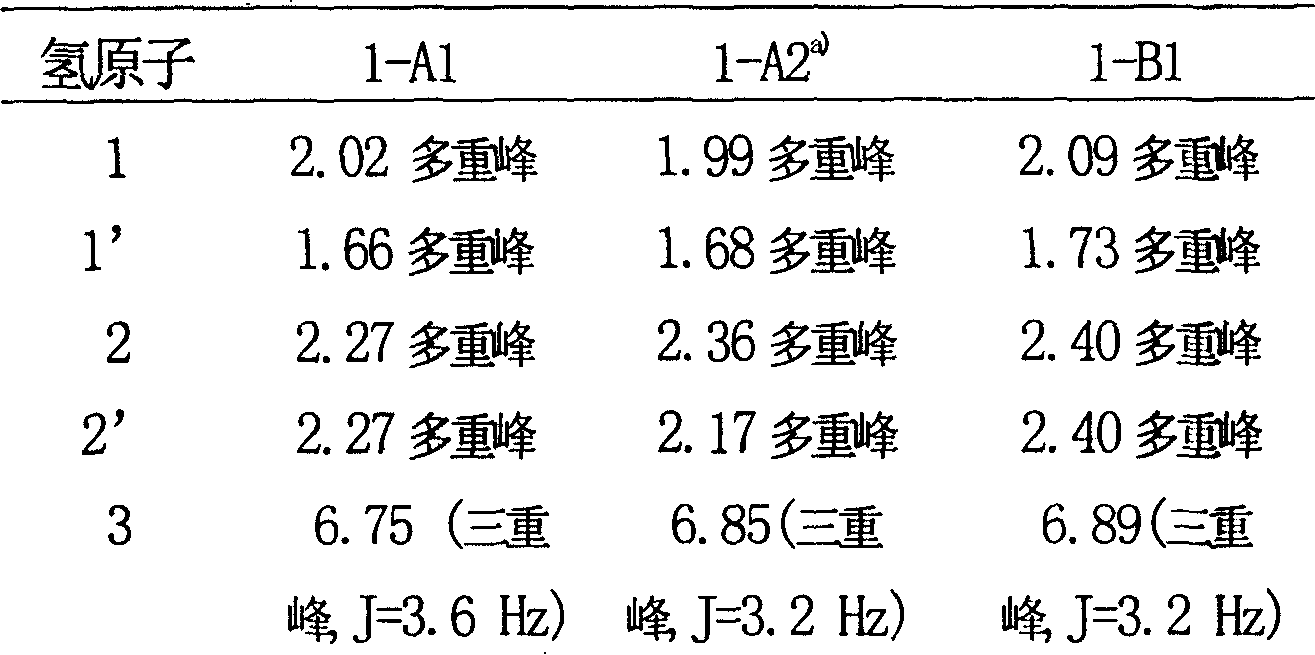
[0031] Physical and spectral data of compound 1-A2: colorless needles; melting point: 167-169°C; [α] D 20 : +41.0°(chloroform; c0.25); UVλ max (Chloroform) nm: 219; IR v max KBr (cm -1 ): 1777, 1741, 1679; Electrospray mass spectrometry ESI-MS (m / z): 291 [M+H] + ; High-resolution electrospr...
Embodiment 3
[0032] Example 3: Preparation of Compound 1-B1
[0033] With embodiment 1, obtain 89.0 gram ethyl acetate extracts from 5.0 kilograms of drying Heiziwu. The extract was chromatographed on 800 g of 100-200 mesh silica gel column, and chloroform-methanol (50:0-0:1) gradient eluted. The same fractions were combined by thin-layer chromatography detection, and the part of the extract containing compound 1-B1 was purified by preparative thin layer (60-90° petroleum ether-ethyl acetate 4:1) followed by Sephadex LH-20 column chromatography ( Methanol elution) to finally obtain compound 1-B1 (23mg).
[0034] Colorless needle crystals; melting point: 172-174°C; [α] D 20 : -62.0° (acetone; c0.20); UVλ max (Acetone) nm: 222; IRv max KBr (cm -1 ): 3325, 1774, 1745, 1671, 1428; Electrospray mass spectrometry ESI-MS (m / z): 277 [M+H] + ; High-resolution electrospray mass spectrometry HR-ESI-MS (m / z): 277.1050 (calculated value [C 15 h 16 o 5 +H] + 277.1031); 13 C and 1 The H N...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com