Tumor targeting nano-micelle based on hyaluronic acid as well as preparation and application of tumor targeting nano-micelle
A hyaluronic acid, tumor targeting technology, applied in the field of medicine, can solve the problems of few nanomaterials and nano dosage forms, lack of good targeting, safe nano-delivery technology platform, single function, etc., to enhance the anti-tumor effect. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1: the synthesis of docetaxel intermediate DTX-SS
[0047] The synthetic route is as follows:
[0048]
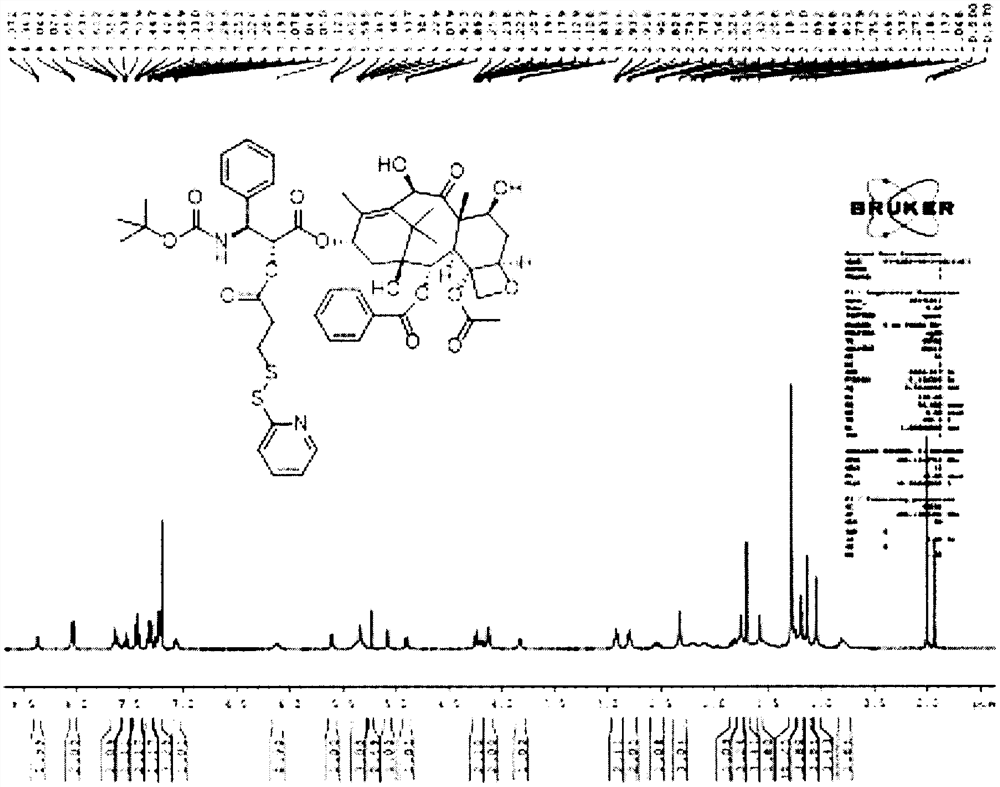
[0049] Dissolve 500mg of docetaxel, 160mg of 3-(pyridin-2-yldisulfanyl)propionic acid, and 23mg of 4-dimethylaminopyridine in 24mL of anhydrous dichloromethane. After fully dissolving, add 134μL of N,N - Diisopropylcarbodiimide, reacted in an oil bath at 25°C for 3 hours. Then dilute the reaction system with 50mL of dichloromethane, with 150mL of NH4 Cl aqueous solution extracted the diluted system twice, collected the organic phase, then extracted the organic phase with saturated NaCl aqueous solution, collected the organic phase, and finally used anhydrous NaCl 2 SO 4 The organic phase was dried, spin-dried, and then the product was purified by column chromatography to finally obtain 294 mg of the docetaxel intermediate. The prepared docetaxel intermediate 1 H-NMR spectrum such as figure 1 shown.
Embodiment 2
[0050] Embodiment 2: the synthesis of paclitaxel intermediate PTX-SS
[0051] The synthetic route is as follows:
[0052]
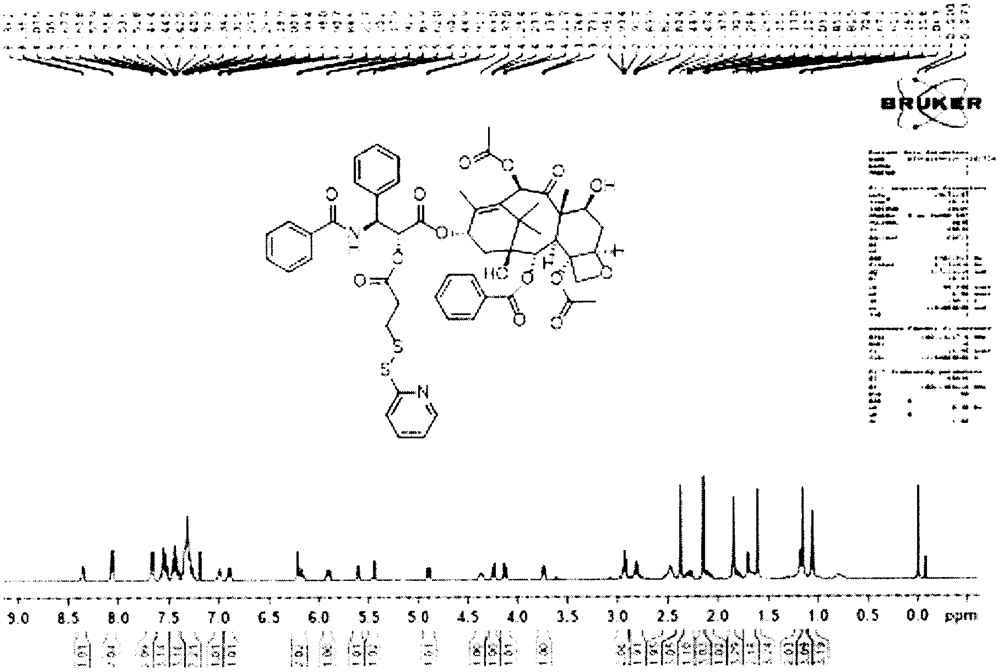
[0053] Dissolve 100mg of paclitaxel, 30mg of 3-(pyridin-2-yldisulfanyl)propionic acid, and a catalytic amount of 4-dimethylaminopyridine in 5mL of anhydrous dichloromethane, and add dropwise 30μL of N,N' after fully dissolving -Diisopropylcarbodiimide, react at room temperature for 3h. The system was diluted with dichloromethane, extracted twice with saturated ammonium chloride, washed once with saturated NaCl, then dried with anhydrous sodium sulfate, spin-dried the solvent under reduced pressure, and used dichloromethane / methanol=90 / 1, 200-300 mesh silica gel Purified by column chromatography to obtain 100 mg of paclitaxel intermediate. Paclitaxel intermediates prepared 1 H-NMR spectrum such as figure 2 shown
Embodiment 3
[0054] Embodiment 3: the synthesis of podophyllotoxin intermediate PODO-SS
[0055] The synthetic route is as follows:
[0056]
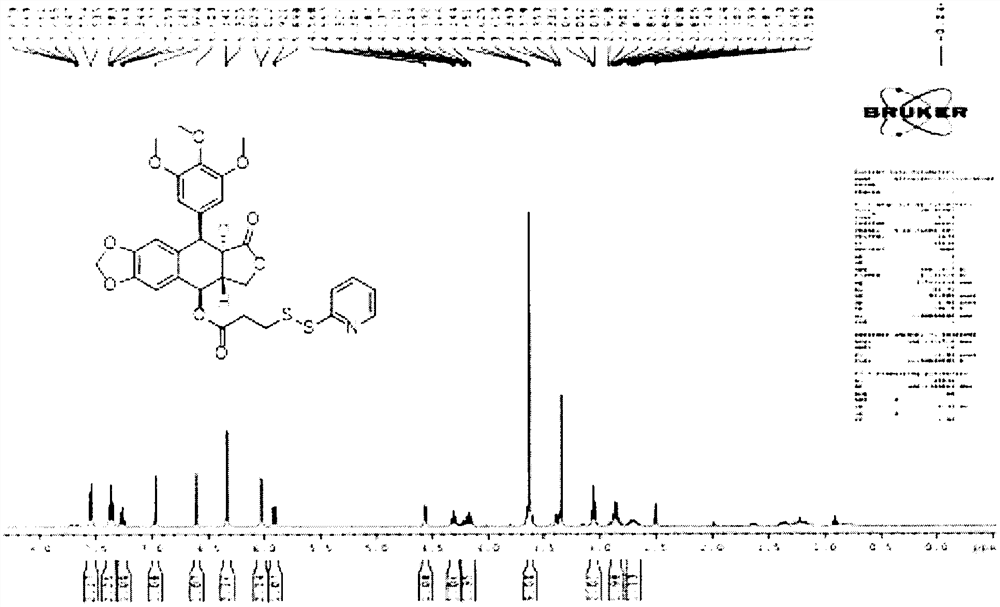
[0057] Dissolve 200mg of podophyllotoxin, 160mg of 3-(pyridin-2-yldisulfanyl)propionic acid, and 70mg of 4-dimethylaminopyridine in 5mL of anhydrous dichloromethane, and dropwise add 150μL of N,N'- Diisopropylcarbodiimide, react at room temperature for 50min. The system was diluted with dichloromethane, extracted twice with saturated ammonium chloride, washed once with saturated NaCl, dried with anhydrous sodium sulfate, spin-dried the solvent under reduced pressure, and used petroleum ether / ethyl acetate=4 / 1, 200-300 mesh Silica gel was purified by column chromatography to obtain 230 mg of a podophyllotoxin intermediate. The prepared podophyllotoxin intermediate 1 H-NMR spectrum such as image 3 shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com