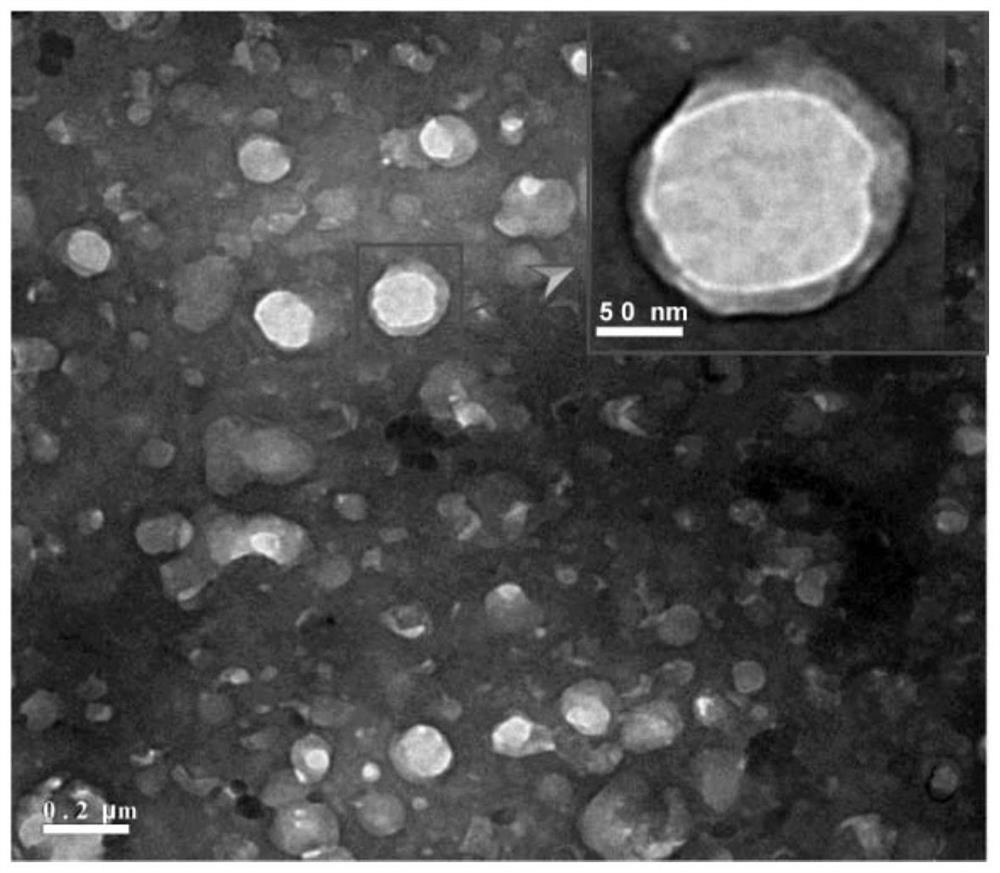
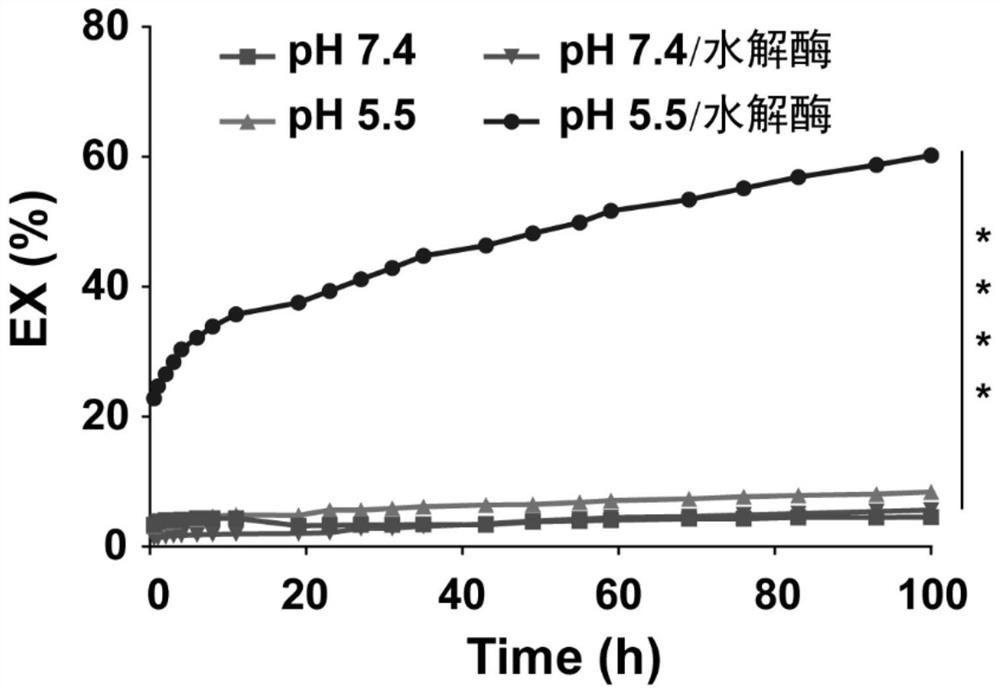
Podophyllotoxin lipid derivative, nano carrier, preparation method thereof, and application of podophyllotoxin lipid derivative and nano carrier in tumor treatment
A technology of lipid nanocarriers and lipid derivatives, applied in the direction of nanotechnology, nanotechnology, nanomedicine, etc., can solve the problems of poor solubility, unsuccessful, toxic clinical applications, etc., to improve solubility, improve side effects, Effect of increasing cellular uptake content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058]
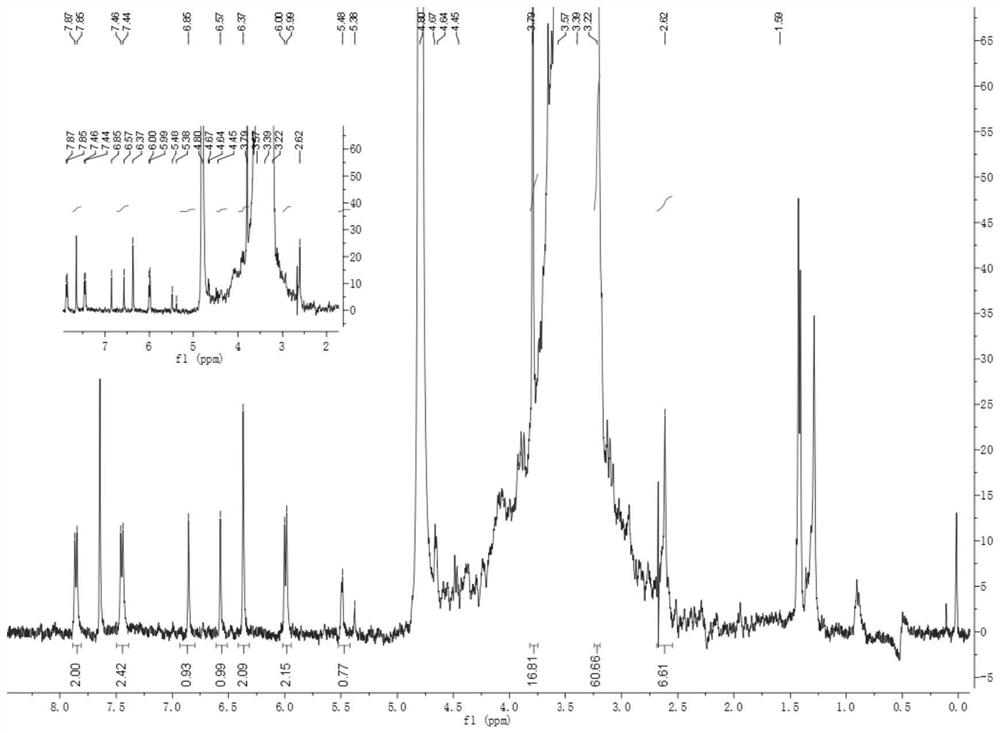
[0059] Take N-4β-((4-methylpiperazine-1-methyl)benzamide)-4'-desmethyl epipodophyllotoxin 2a 1.0g (1eq) and 1-tetradecylcarbamate 3a Put 1.1g (2.8eq) in a 50mL round bottom flask, add 8mL tetrahydrofuran solvent, and then add 1.0g (1.2eq) of diethyl azodicarboxylate and 0.8g (1.2eq) of triphenylphosphine under the action of The reaction was stirred at room temperature for 1.5 h. After the reaction was complete as detected by TLC, the solution was evaporated to dryness under reduced pressure to obtain 1.73 mg of a yellow powder, namely compound 1a, with a yield of 48%.
Embodiment 2
[0061]
[0062] Take N-4β-((4-methylpiperazine-1-methyl)benzamide)-4'-desmethyl epipodophyllotoxin 2a 0.5g (1eq) and 1-tetradecylcarbamate 3a 2.5g (2.8eq) in a 50mL round bottom flask, add 5mL dimethylformamide solvent, then add N,N'-diisopropylcarbodiimide 5ml (1.0eq) and 1-hydroxybenzotri Azole (1.2eq) was reacted at 0°C for 2h under stirring. After the reaction was complete as detected by TLC, the solution was evaporated to dryness under reduced pressure to obtain 2.08mg of yellow powder, namely compound 1b, with a yield of 59%.
Embodiment 3
[0064] Take N-4β-((4-methylpiperazine-1-methyl)benzamide)-4'-desmethylepipodophyllotoxin 2a10 g (1eq) and 1-tetradecylcarbamate 3a10 g (1.8eq) in a 50mL round bottom flask, add 10mL dimethylformamide solvent, then add 2-(7-azobenzotriazole)-N,N,N',N'-tetramethyl 17.8g (1.2eq) of urea hexafluorophosphate and 3ml (1.0eq) of N,N-diisopropylethylamine were stirred and reacted at 0°C for 1h. After the reaction was complete as detected by TLC, the solution was evaporated to dryness under reduced pressure to obtain yellow 13.23mg of the powder is compound 1b, and the yield is 87%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com