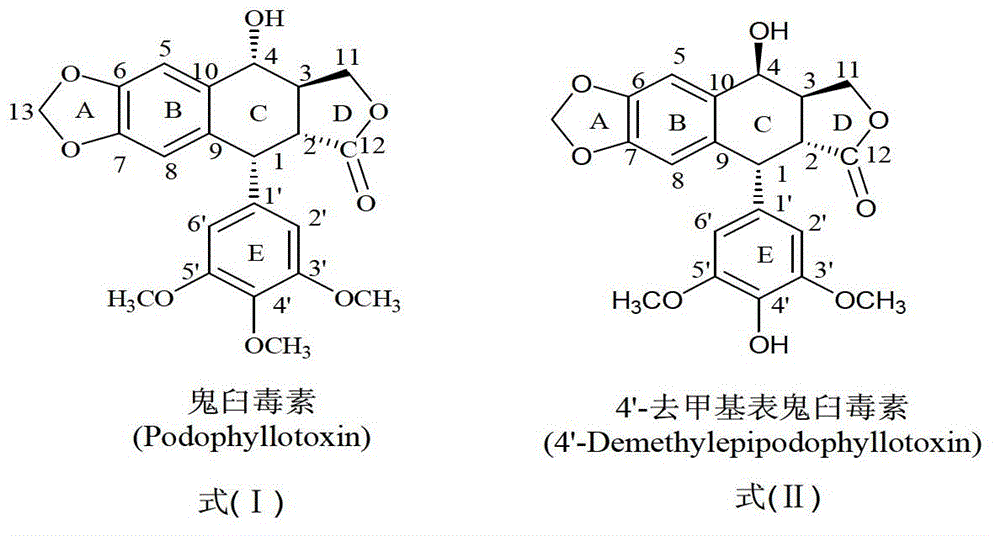
Esterified podophyllum derivative with antitumor activity and preparation method and usage of esterified podophyllum derivative
A technology of anti-tumor activity and derivatives, applied in the direction of anti-tumor drugs, organic active ingredients, medical preparations containing active ingredients, etc., can solve the problems of low anti-tumor activity and limited use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
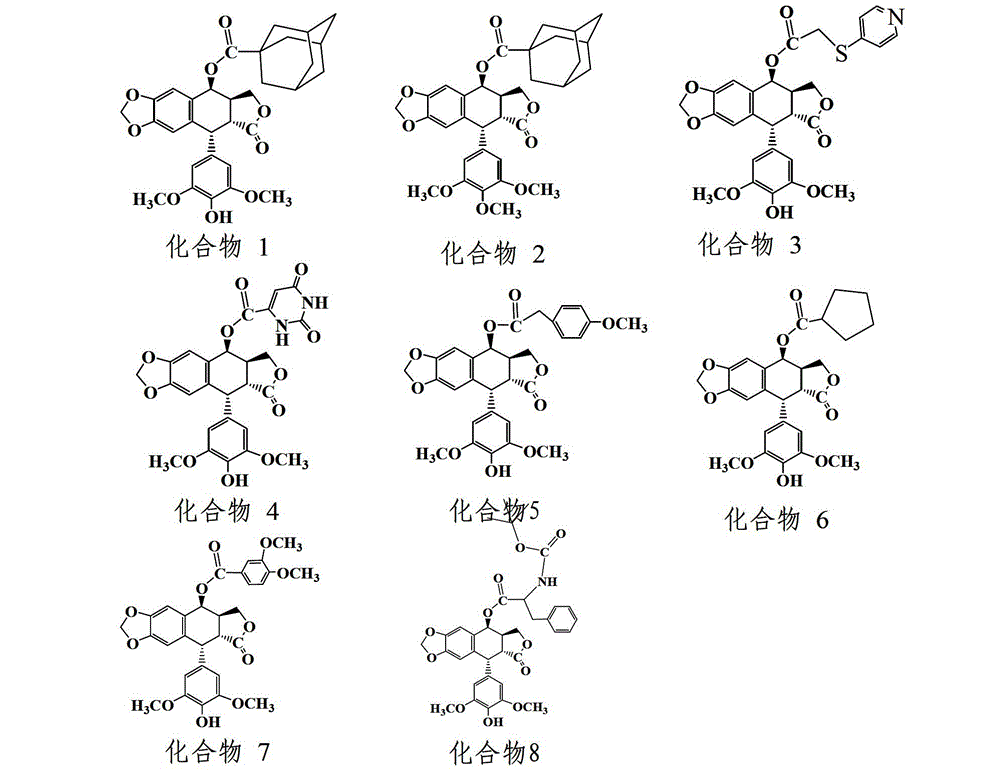
[0036] Example 1 Synthesis and purification of 4-O-(adamantanecarboxylic acid-1)-4-deoxy-4'-desmethyl epipodophyllotoxin (compound (1))
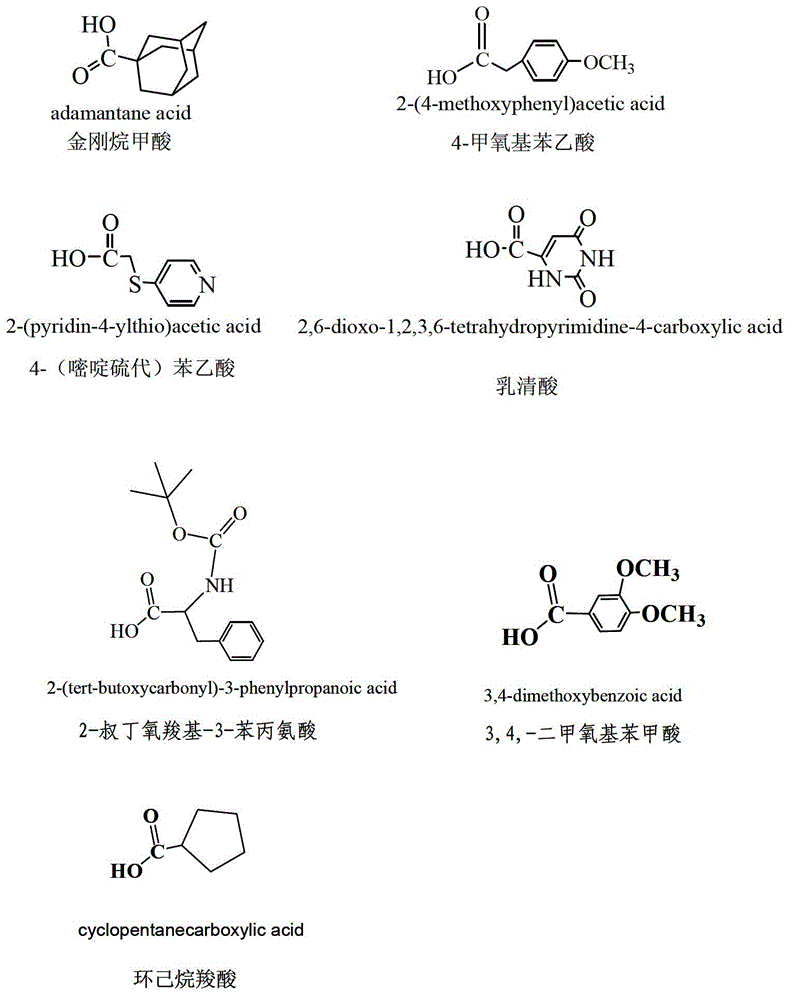
[0037] (1) Synthesis of 4-O-(adamantanecarboxylic acid-1)-4-deoxy-4'-desmethyl epipodophyllotoxin: Weigh 207mg of 4'-desmethyl epipodophyllotoxin and 270mg of adamantanecarboxylic acid respectively In a plate, vacuum-dry at 45°C for 2 hours; under the protection of nitrogen, add the dried 4'-desmethyl epipodophyllotoxin and adamantanecarboxylic acid and 1236 mg of dicyclohexylcarbodiimide into a four-necked bottle, and then add 10ml of dried dichloromethane, stirred and reacted for 30min, then added 10mg of 4-dimethylaminopyridine to the reaction system, and reacted at room temperature 25°C for 24 hours; after the reaction was completed, filter the reaction solution with filter paper to remove insoluble matter, add 100ml of deionized water, keep the organic phase, repeat twice, then back-extract the aqueous phase obtained in the previous ste...
Embodiment 2
[0042] Example 2 Synthesis and purification of 4-O-(adamantanecarboxylic acid-1)-4-deoxypodophyllotoxin (compound (2))
[0043] (1) Synthesis of 4-O-(adamantanecarboxylic acid-1)-4-deoxypodophyllotoxin: Weigh 207mg of podophyllotoxin and 510mg of adamantanecarboxylic acid respectively in a plate, dry in vacuum at 45°C for 2 hours; , add the dried podophyllotoxin, adamantanecarboxylic acid and 1236mg of dicyclohexylcarbodiimide into a four-necked flask, then add 10ml of dried dichloromethane, stir for 30min, and then add 10mg of 4-dichloromethane to the reaction system Methylaminopyridine, react at 20°C for 48 hours; after the reaction is completed, filter the reaction solution with filter paper to remove insoluble matter, add 100ml deionized water, keep the organic phase, repeat twice, and then use dichloromethane to dilute the obtained solution in the previous step. The aqueous phase was back-extracted, the organic layers were combined, dried overnight with anhydrous sodium s...
Embodiment 3
[0049] Example 3 Synthesis and purification of 4-O-(4-(pyrimidinethio)acetic acid-1)-4-deoxy-4'-desmethyl epipodophyllotoxin (compound (3))
[0050] (1) Synthesis of 4-O-(4-(pyrimidinethio)acetic acid-1)-4-deoxy-4′-desmethyl epipodophyllotoxin: Weigh 200mg of 4′-desmethyl epipodophyllotoxin respectively and 504mg of 4-(pyrimidinylthio)acetic acid in a plate, and vacuum-dry at 45°C for 2 hours; In the four-neck flask, add 2ml of dried dichloromethane and 1.6ml of triethylamine to react for 30min, add 5mg of 4-dimethylaminopyridine to the reaction system, and react at 25°C for 24 hours. After completion of the reaction, filter the reaction solution with filter paper to remove insoluble matter, add 100ml of deionized water, keep the organic phase, repeat twice, then use dichloromethane to back-extract the aqueous phase obtained in the previous step, combine the organic layers, and use Dry overnight with anhydrous sodium sulfate, spin dry to obtain the crude product of 4-O-(4-(py...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The inside diameter of | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com