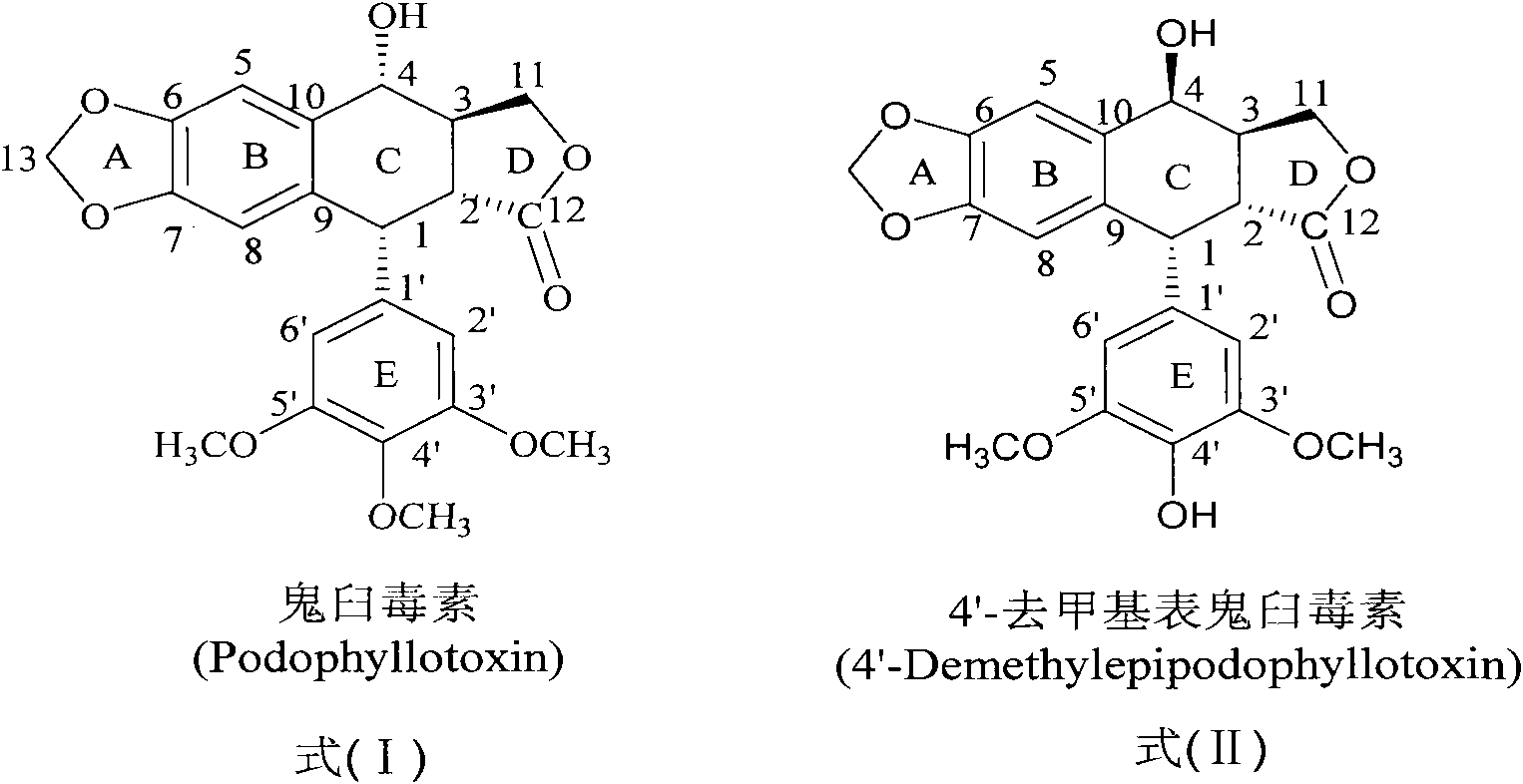
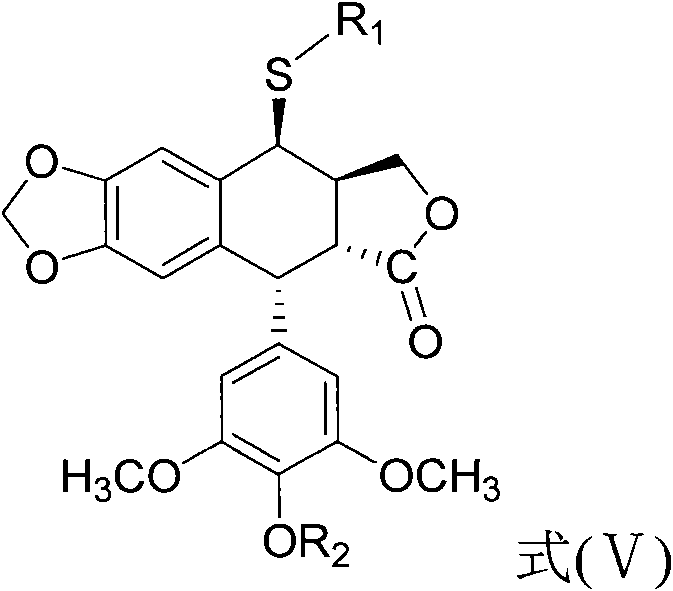
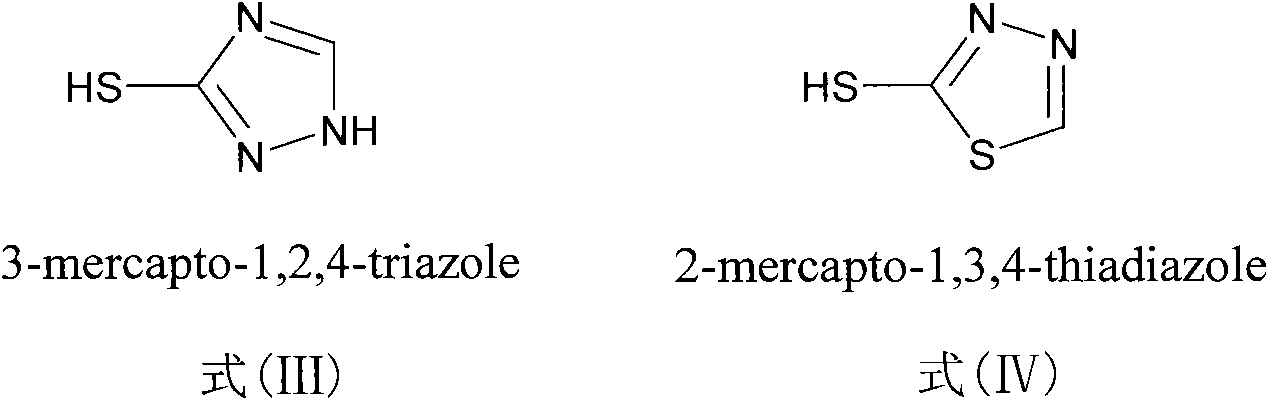Sulfur-substituted podophyllum derivative and synthetic method and application thereof
A podophyllotoxin and reaction technology, applied in the field of podophyllin derivatives, can solve problems such as toxic side effects and poor bioavailability, and achieve the effects of reducing toxic side effects, improving anti-tumor activity, and improving anti-tumor efficacy.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1 Synthesis and purification of 4-S-(1,2,4-triazole-3)-4-deoxypodophyllotoxin (compound (1))
[0036] (1) Synthesis of 4-(1,2,4-triazole-3)-4-deoxypodophyllotoxin: take 414mg (1mmol) of podophyllotoxin, 101mg (1mmol) of triazole, and vacuum-dry for 1h , using 15ml of trifluoroacetic acid as a solvent in an ice bath, stirring in vacuum (600 rpm) at 4°C for 1 h, using chloroform acetone as a developer, and detecting the end point of the reaction. Concentrate under reduced pressure to nearly dryness, dissolve in ethyl acetate, and wash three times with 10 ml of deionized water, collect the ethyl acetate layer, dry with anhydrous sodium sulfate, and concentrate to obtain the crude product.
[0037] (2) Separation and purification of 4-(1,2,4-triazole-3)-4-deoxypodophyllotoxin:
[0038] Separation and purification using silica gel column chromatography and gel column chromatography:
[0039] (A) Use a normal phase silica gel column (normal phase silica gel: China Q...
Embodiment 2
[0042] Example 2 Synthesis and purification of 4-S-(1,3,4-thiadiazole-2)-4-deoxypodophyllotoxin (compound (2))
[0043] (1) Synthesis of 4-S-(1,3,4-thiadiazole-2)-4-deoxypodophyllotoxin: get 414mg (1mmol) of podophyllotoxin, 118mg (1mmol) of triazole, vacuum Dry for 1 hour, use 15 ml of trifluoroacetic acid as a solvent under ice bath conditions, stir in vacuum (600 rpm) at 4°C for 1.5 hours, use chloroform acetone as a developer, and detect the end point of the reaction. Concentrate under reduced pressure to nearly dryness, dissolve in ethyl acetate, and wash three times with 10 ml of deionized water, collect the ethyl acetate layer, dry with anhydrous sodium sulfate, and concentrate to obtain the crude product.
[0044] (2) Separation and purification of 4-S-(1,3,4-thiadiazole-2)-4-deoxypodophyllotoxin:
[0045] Separation and purification using silica gel column chromatography and gel column chromatography:
[0046] (A) Use a normal phase silica gel column (normal phase s...
Embodiment 3
[0049] Example 3 Synthesis and purification of 4-S-(1,2,4-triazole-3)-4-deoxy-4'-desmethyl epipodophyllotoxin (compound (3))
[0050] (1) Synthesis of 4-S-(1,2,4-triazole-3)-4-deoxy-4'-demethyl epipodophyllotoxin: get 400mg (1mmol) of podophyllotoxin, 101mg ( 1mmol) of triazole, vacuum-dried for 1h, under ice-bath conditions, with 15ml of trifluoroacetic acid as solvent, stirred in vacuum (800 rpm) at -4°C for 3h, using chloroform acetone as developer, and detected the end point of the reaction. Concentrate under reduced pressure to nearly dryness, dissolve in ethyl acetate, and wash three times with 10 ml of deionized water, collect the ethyl acetate layer, dry with anhydrous sodium sulfate, and concentrate to obtain the crude product.
[0051] (2) Isolation and purification of 4-S-(1,2,4-triazole-3)-4-deoxy-4'-desmethyl epipodophyllotoxin:
[0052] Separation and purification using silica gel column chromatography and gel column chromatography:
[0053] (A) Use a normal ph...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The inside diameter of | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com