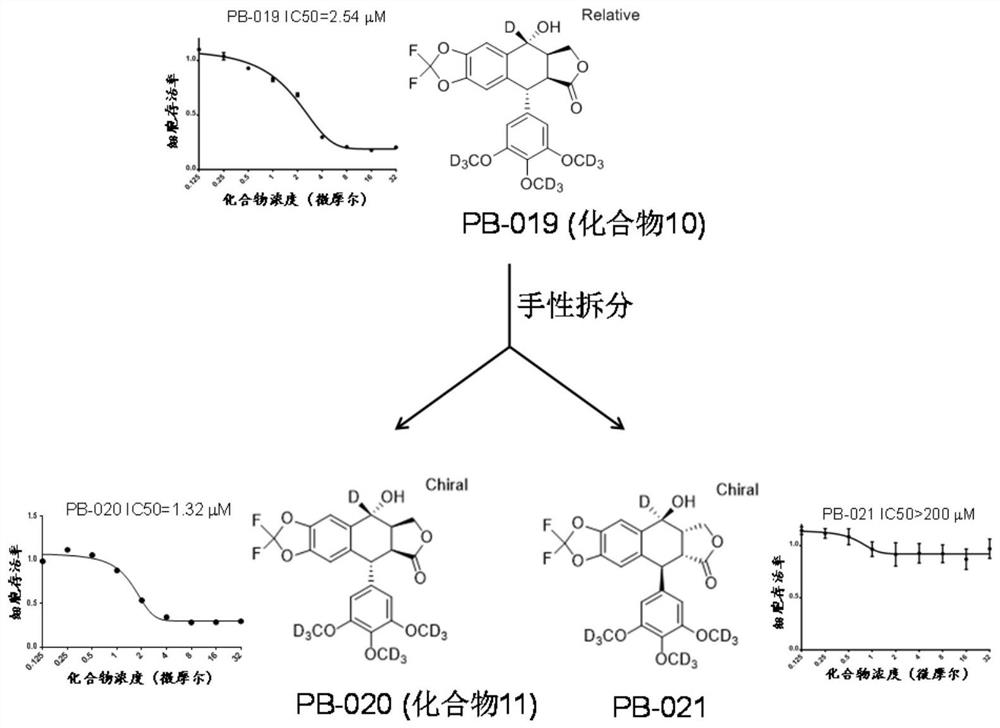
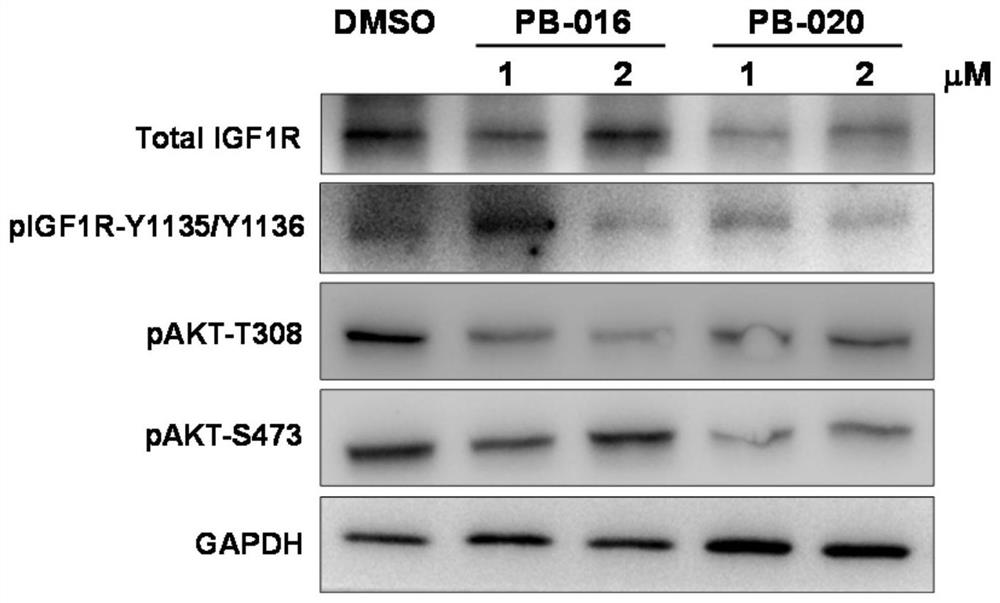
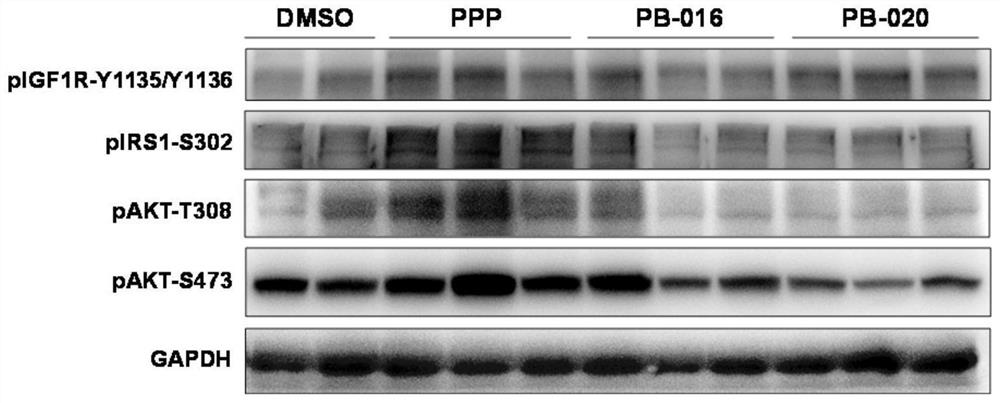
Novel small molecule inhibitor of insulin-like growth factor-1 receptor and application of novel small molecule inhibitor
A technology of small molecule inhibitors and growth factors, applied in medical preparations containing active ingredients, organic active ingredients, organic chemistry, etc., can solve problems such as poor effect and difficulty penetrating the blood-brain barrier, and achieve long half-life, Effect of good blood-brain barrier permeability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1, relative-(5R,5aS,8aR,9R)-2,2-difluoro-9-hydroxyl-5-(3,4,5-trimethoxyphenyl)-5,8,8a,9 -Tetrahydrofuro[3',4':6,7]naphtho[2,3-d][1,3]-dioxan-6(5aH)-one (compound 1)
[0047]
[0048] Step 1), synthesis of 4-vinyldihydrofuran-2(3H)-one (intermediate 1.1):
[0049] To a mixture of 2-butene-1,4-diol (206.4g, 2.34mol, 1.0eq) and triethylorthoacetate (546.7g, 3.4mol, 1.4eq) was added catalytic hydroquinone (54.00g , 0.49mol, 0.2eq), the mixture was heated to 120°C. Ethanol was continued to be distilled off until no more ethanol was produced, the reaction temperature was raised to 150°C and the reaction mixture was stirred for 48 hours. Intermediate 1.1 was collected by vacuum distillation (70-75 °C, 2-3 mmHg) as a colorless oil (170.0 g, 65% yield). 1 HNMR (400MHz, CDCl 3 )δ (ppm) 2.36 (dd, J = 17.4Hz, 8.7Hz, 1H), 2.69 (dd, J = 17.7Hz, 8.4Hz, 1H), 3.19-3.29 (m, 1H), 4.01-4.14 (m, 1H), 4.43-4.47(m, 1H), 5.17-5.23(m, 2H), 5.75-5.84(m, 1H).
[0050] Step 2), re...
Embodiment 2
[0062] Example 2, relative-(5R,5aS,8aR,9R)-2,2-difluoro-8-oxa-9-(3,4,5-trimethoxyphenyl)-5,5a,6, 8,8a,9-Hexahydrofuro[3',4':6,7]naphtho[2,3-d][1,3]-dioxan-5-yl acetate (compound 2)
[0063]
[0064] Compound 1 (1.15g, 2.55mmol, 1.0eq) and dichloromethane (30ml) were continuously stirred and mixed, and Et 3 N (770mg, 7.6mmol, 3.0eq) and DMAP (310mg, 2.55mmol, 1.0eq), then AcCl (400mg, 5.1mmol, 2.0eq) was added under ice bath, and the mixture was stirred at ambient temperature for 12 hours. LiAl(OtBu) was added dropwise 3 (200ml, 197mmol, 2.0eq), then gradually heated to ambient temperature overnight. React with saturated NH 4 Quenched with Cl, the aqueous phase was further extracted with dichloromethane. The combined organic phases were washed with saturated brine and dried over anhydrous sodium sulfate. The residue was recrystallized from petroleum ether / ethyl acetate to obtain compound 2 (810 mg, yield 65%) as a white solid. 1 HNMR (400MHz, CDCl 3 )δ(ppm)7.02(s,1H),...
Embodiment 3
[0065] Example 3, relative-(5R,5aS,8aR,9R)-2,2,9-trifluoro-5-(3,4,5-trimethoxyphenyl)-5,8,8a,9-tetrahydrofuran And[3',4':6,7]naphtho[2,3-d][1,3]-dioxan-6(5aH)-one (Compound 3)
[0066]
[0067] Compound 1 (700mg, 1.60mmol, 1.0eq) and dichloromethane (20ml) were continuously stirred and mixed, diethylaminosulfur trifluoride (520mg, 3.20mmol, 2.0eq) was added dropwise, and the mixture was stirred at ambient temperature for 12 Hour. React with saturated NaHCO 3 Quenched and stirring was continued for 30 minutes. After separation of the organic phase, the aqueous phase was further extracted with dichloromethane. The combined organic phases were washed with saturated brine and dried over anhydrous sodium sulfate. The residue was purified by silica gel column chromatography (200-300 silica gel, dichloromethane:methanol=100 / 1-50 / 1) to obtain compound 3 (130 mg, yield 18%) as a white solid. 1 HNMR (400MHz, CDCl 3 )δ(ppm)7.27(s,1H),6.66(s,1H),6.44(s,2H),5.49-5.34(d,J=50.8Hz,1H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com