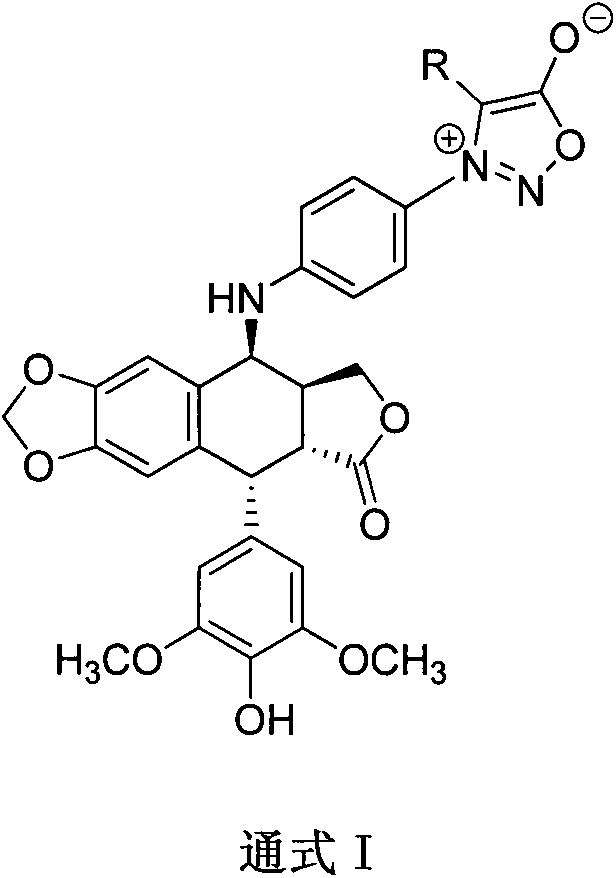
4-sydnone substituted phenyl epipodophyllotoxin derivative and preparation method and application thereof
A technology of epipodophyllotoxin and sterone, which is applied in the fields of 4-sterone substituted anilino-epipodophyllotoxin derivatives and the fields of preparation and application thereof, and can solve the problems of unstopped podophyllotoxin and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
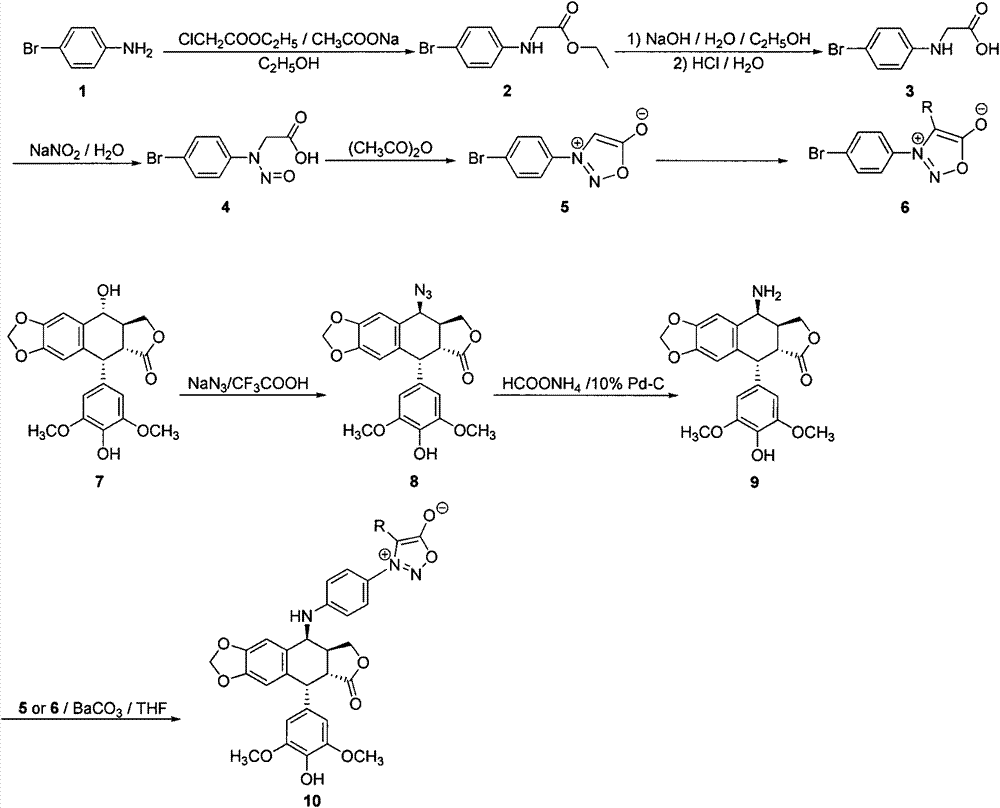
Embodiment 1
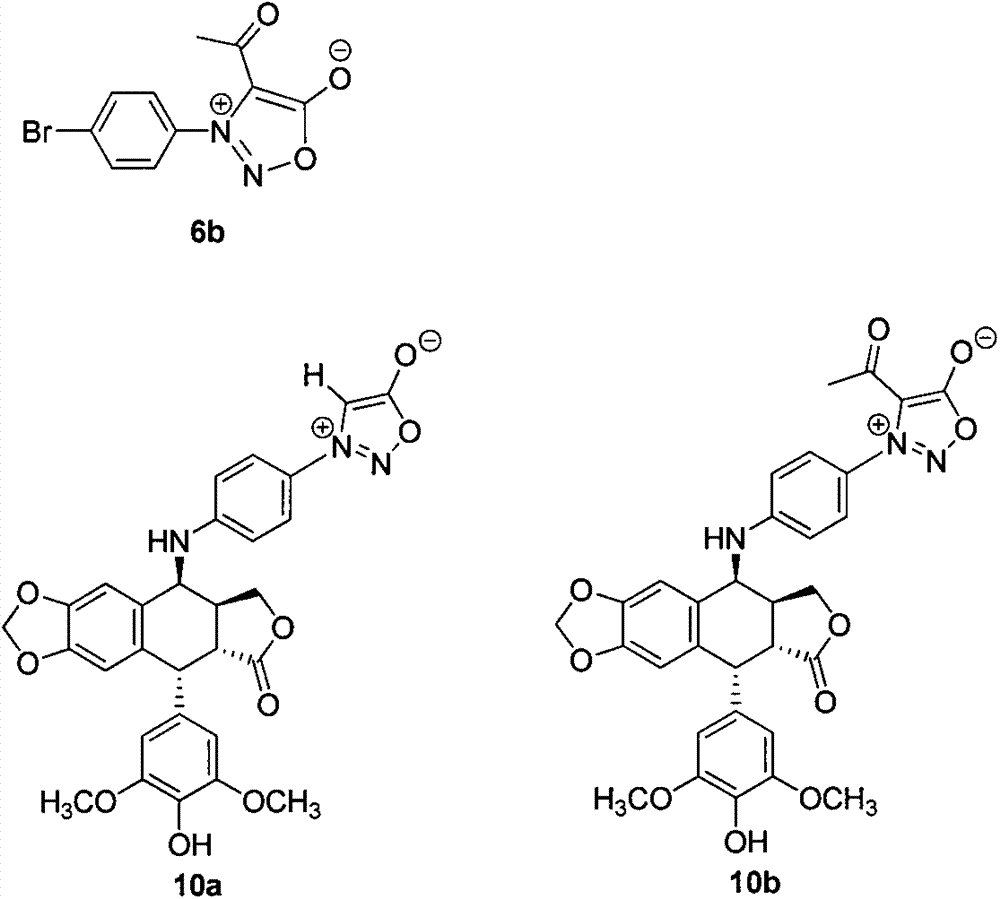
[0019] Synthesis of Compound 10a:
[0020] Compound 5 is prepared from compound 1 by the method of literature (Org.Commun., 2010, 30). Compound 5 can be prepared into compound 6 with various structures through acylation, halogenation, sulfonylation and other reactions.
[0021] Compound 9 was prepared from compound 7 by the method of literature (Chem.Pharm.Bull., 2008, 831-834).
[0022] Dissolve 0.42g (1.0mmol) of 4-amino-4-deoxy-4'-demethylepipodophyllotoxin (9) in 10mL of tetrahydrofuran with stirring, and cool the reaction solution to 0°C in an ice bath. At this temperature, 0.40 g (2.0 mmol) of barium carbonate and 0.36 g (1.5 mmol) of compound 5 were added to the reaction flask, the ice bath was removed, and the reaction was stirred at room temperature for 10 h under nitrogen protection. After completion of the reaction, filter with suction, add 20 mL of water to the filtrate, extract with ethyl acetate, dry over anhydrous sodium sulfate, and concentrate the solvent und...
Embodiment 2
[0024] Synthesis of Compound 10b:
[0025] Compound 6b was prepared from compound 5 by the method of literature (Heterocycles, 2008, 91).
[0026] Dissolve 0.42g (1.0mmol) of 4-amino-4-deoxy-4'-demethylepipodophyllotoxin (9) in 10mL of tetrahydrofuran with stirring, and cool the reaction solution to 0°C in an ice bath. At this temperature, 0.40 g (2.0 mmol) of barium carbonate and 0.43 g (1.5 mmol) of compound 6b were added to the reaction flask, the ice bath was removed, and under the protection of nitrogen, the reaction was stirred at room temperature for 10 h. After completion of the reaction, filter with suction, add 20 mL of water to the filtrate, extract with ethyl acetate, dry over anhydrous sodium sulfate, and concentrate the solvent under reduced pressure to obtain a crude product, which is purified by column chromatography (petroleum ether-ethyl acetate gradient elution) to obtain the product, the product is Pale yellow solid, yield 63%; Mp192-194°C (d); -86.0 (c=...
experiment example
[0028] Experimental example: the compound designed by the present invention has inhibitory effect on tumor cells
[0029] Method: Using MTT method
[0030] The in vitro anti-tumor activity of the target compound on HeLa cells (human cervical cancer cells, Henrietta Lacks cancer cell line) and SKOV3 cells (human ovarian cancer cells, ovarian epithelial carcinoma cell line) was tested by MTT (thiazolium blue) method. The test results are shown in Table 1, and the values in the table are average values of three times (n=3). The results of the activity test show that the synthesized compound has in vitro activity against two kinds of human tumor cells, and this type of compound has the value of further research.
[0031] The in vitro antitumor activity of table 1 compound 10a and 10b
[0032]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com